Irene Y.T. Chang, Derk Joester
______________________________________________________________
As man-made materials become more similar to the biological structures that inspire them, they increasingly combine nano-sized hard and soft, synthetic and biological components. This creates new challenges for characterization, especially in those materials where water is an integral part of the structure. Cryogenic sample preparation and imaging is often necessary for such specimens. Imaging of cryo-fixed (vitrified), freeze-fractured samples by cryo-SEM is particularly efficient. However, the propagation of the fracture plane is unpredictable at best, and frequently the fracture surface fails to reveal the interface of interest. Sample preparation of hydrated composite systems, for example the sea urchin embryo with its mineralized endoskeleton, is further complicated by the large hardness contrast between the organic and the biomineral phase.
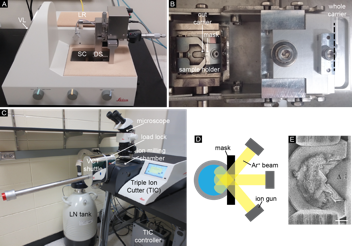
We have developed a new cryogenic sample preparation workflow, cryo triple ion gun milling (CryoTIGM™), for cryo-planing and imaging large areas of frozen-hydrated samples. The method, based on a Leica TIC3X ion miller, includes a vacuum load lock and a vacuum-cryo-transfer shuttle that enable cryo-transfer of a vitrified sample (Fig. 1). Specimens are high-pressure frozen, trimmed with a cryo-saw, and cryo ion-milled with three broad Ar+ beams. This process creates a very large and smooth cross section in the sample. The ion-milled sample is then freeze-etched and metal-coated before imaged using cryo-SEM.
|
| Figure 1. Experimental setup of CryoTIGM™. (A) The cryo-saw used for trimming sample carriers. DS = diamond saw, LR = LN reservoir, SC = sample compartment, VL = VCT loading dock. (B) Top view of the sample compartment. A sample carrier is trimmed (along the dashed line) and transferred to the sample holder. (C) CryoTIGM™ tool with attached cryo-vacuum-transfer-shuttle. (D) Schematic drawing of CryoTIGM™ milling process. (E) A frozen hydrated sample after milling (3.0 kV, 1.0 mA/gun, -120oC, 2 h) shows a triangular cryo-planed area of 700,000 µm2 (Δ). |
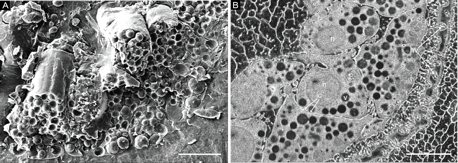
A direct comparison of samples prepared by conventional freeze fracture and CryoTIGM™ reveals that 1) surfaces prepared by CryoTIGM™ are much smoother; 2) cellular and organellar details are observed at comparable high resolutions with good contrast; 3) most importantly, additional ultrastructural features can be revealed. For example, in CryoTIGM™-prepared sea urchin embryo samples (Fig. 2), tight contacts among neighboring ectodermal cells, membrane tethers extended from ectodermal cells to the hyaline layer, and vesicles/granules within the hyaline layer can be easily identified.
|
| Figure 2. Cryo-SEM images of sea urchin embryos prepared by freeze fracture (A) and CryoTIGM™ (B), showing part of the thickened vegetal plate. hl: hyaline layer; hv: vesicles within the hyaline layer; ic: intercellular contacts; n: nucleus; t: tethers; v: cytoplasmic vesicle. |
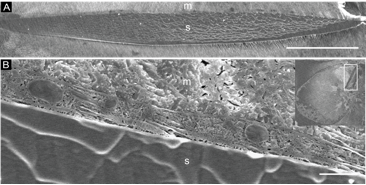
Furthermore, preliminary results show that CryoTIGM™ can generate high-quality sections of cell-endoskeleton interfaces of the sea urchin embryo (Fig. 3). The membranes of the syncytium that envelope the endoskeleton appear well-defined. Numerous organelles are observed within the syncytial compartment, indicating excellent preservation of cellular ultrastructure. These results suggest that CryoTIGM™ is a promising new tool for interfacial studies of hybrid hard/soft materials.
|
Figure 3. Cryo-SEM of sectioned endoskeleton of sea urchin embryos prepared by CryoTIGM™. (A) Plane of intercept of the ion beams and the endoskeleton. (B) The cross-section of a syncytium enveloping the endoskeleton. Various organelles are observed within it. The inset shows an ion-milled whole embryo, whose endoskeleton is enclosed by a rectangle and shown close-up in panel A. |
Home | Contact Us | Sites A-Z









