Karen Derocher, Robert Free, Lyle Gordon, Derk Joester
______________________________________________________________
Dental caries, or tooth decay, is one of the most prevalent forms of infectious disease in the world, affecting nearly 100% of individuals at some point in their lives1. Caries commonly advances via acid demineralization of tooth enamel by bacteria-produced acids in the oral cavity. We explore the role that the nano-structure and phase composition of enamel plays in the development of carious lesions. Through a combination of in vitro and in vivo experiments on human and non-human enamel systems, we aim to elucidate the mechanisms through which demineralization progresses and to leverage this understanding to reinforce human enamel against carious attack.
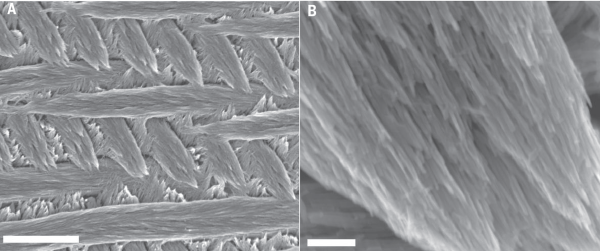
Enamel is a hierarchical biocomposite with structural organization across multiple length scales (see figure 1 below). At the nano-scale, the system is composed of hydroxylapatite (OHAp) [Ca10(PO4)6(OH)2] single-crystal nanowires between 25 and 50 nm across and many microns in length. Between these densely packed nanowires exists thin layers of interphase mineral that is either nanocrystalline or completely amorphous2. On the micron-scale, groups of ~104 nanowires are bundled into similarly-oriented enamel rods, which are themselves interwoven in a 3-dimensional pattern and interspersed with less well-ordered interrod enamel.
|
| Figure 1. Hierarchical structure of enamel. (A) Enamel rods and interrod enamel in 3-D weave (scale: 5µm) (B) Single rod illustrating oriented nanowire sub-structure (scale: 250nm). Adapted from Gordon, et. al.2. |
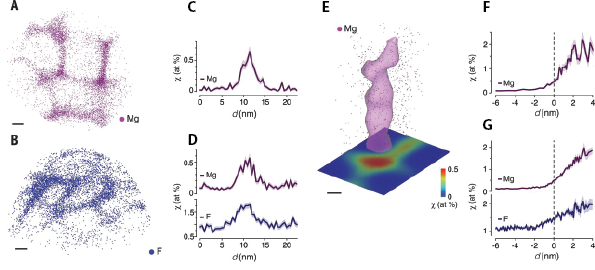
Using the chemical specificity and sub-nanometer spatial resolution of atom probe tomography (APT), (linked to other research page) L. Gordon from our group hasdemonstrated that the susceptibility of enamel systems to acid etching is strongly dictated by the composition of the disordered intergranular phases between OHAp nanowires (Lyle, et al.). Furthermore, this powerful technique allows high-resolution characterization of chemical the distributions at the scale of nanowires, which can provide insights into the phase and structure of enamel in unprecedented detail (figure 2). We now look to extend this powerful characterization technique to explore the evolution of these newly identified and highly relevant interphases under more realistic carious conditions; namely, through in vitro artificial carious lesions in human enamel and in vivo caries models in rats.
|
| Figure 2. Atom Probe Tomography of Enamel Nanowire Clusters. (A) Mg ion positions in mouse outer enamel. (B) F ion positions in fluoride-treated mouse inner enamel. (C, D) Representative concentration profiles across grain boundaries. (E) Isosurface (0.5 atomic %) surrounding Mg-rich multiple grain boundary in (A). (F, G) Representative proxigrams of multiple grain boundaries in (F) mouse outer enamel and (G) fluoride-treated outer mouse enamel. Adapted from Lyle, et. al.2. |
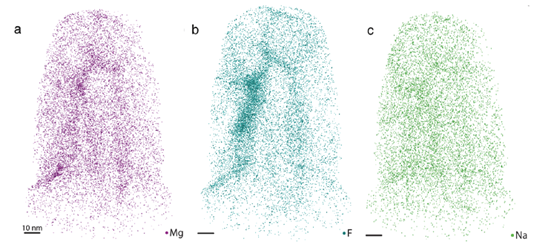
Preliminary APT analysis of human enamel has shown that a Mg-rich amorphous calcium phosphate (ACP) phase also exists in human enamel, consistent with L. Gordon’s findings in rodent enamel. It has also been shown that fluoride ions rapidly diffuse in this amorphous interphase region, co-localizing with magnesium ions (figure 3). Presence of fluoride in this region slows the dissolution of the interphase and increases the enamel’s ability to withstand acidic attack. Current efforts are being made to develop an in vitro caries model to study the chemical and structural changes that occur in the interphase during caries progression. Looking forward, we will analyze the effect of different ions (such as fluoride and iron) on the dissolution and precipitation in enamel. Understanding these processes may eventually lead to better detection and treatment of caries.
|
| Figure 3. Atom probe tomography of human enamel. (A) Mg2+, (B) F-, and (C) Na+ ion positions in human enamel. Co-localization of Mg2+ and F- ions in the interphase regions is evident. |
|
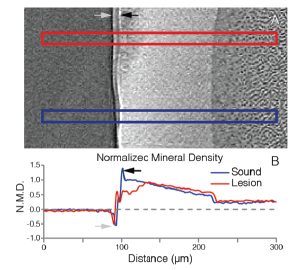
Figure 4: µ-CT reveals lesion mineral density in rat molars. Top: Single slice from x-ray tomographic reconstruction showing integration regions for profiles through a lesion (red) and sound enamel (blue). Bottom: Plot of normalized mineral density from integrated regions. Gray and black arrows indicate phase contrast artifacts and surface lacquer effects. |
Rat models are found frequently in caries research, and rodent enamel is generally accepted as a suitable analog to human enamel3. We aim to leverage similar rodent models to explore the evolution of carious lesions from a materials science perspective. As one example of characterization conducted on lesions produced in rat molars, we rely on x-ray microtomography to reveal the 3-dimensional extent and degree of demineralization in lesions at the micron-scale (figure 4). Correlating these longer-scale characterizations with subsequent atom probe investigations at the nano-scale places our observations in the larger context of lesion development, allowing us to connect the chemical and structural evolution of in vivo dental caries across multiple levels of the material’s hierarchy. We hope such comprehensive investigations across species will yield insights that inform the development of novel preventative treatments for tooth decay.
1 “Oral health” Fact sheet (World Health Organization Media Centre; 2012).
2 Gordon, L.; Cohen, M.; MacRenaris, K.; Pasteris, J.; Seda, T.; Joester, D. Amorphous intergranular phases control the properties of rodent tooth enamel. Science, 2015, 347, 746-750.
3 W. H. Bowen. Rodent model in caries research. Odontology, 2013 101, 9–14.
Home | Contact Us | Sites A-Z









