Ching-Hsuan (Ann) Wu, Derk Joester
______________________________________________________________
Overview
Organisms have evolved unique ways to build endo- and exoskeletal materials that are optimally adapted to their functions. These biomaterials, usually composites of macromolecules (proteins and polysaccharides) and minerals, have a much greater control over polymorph, shape, and architecture than their synthetic counterparts despite the formation occur under ambient conditions. Much could be gained if we could harness this control by influencing the biological crystal growth machinery.
Engineering Single Crystal Growth
One of the fundamental problems is that many biominerals are deposited in complex tissues and as a consequence cannot easily be grown in vitro. A rare exception is the primary mesenchyme cells (PMCs) of sea urchin embryos. The PMCs synthesize single crystalline calcite (CaCO3) endoskeletal elements (spicules) in vivo, and retain this ability to work cooperatively to form spicules even when cultured in vitro. We thus hypothesize that by manipulating the placement of the PMCs in vitro, we can govern the growth of spicules and form single crystalline calcite with desired shapes.
We are developing micro-patterned cell culture substrates for patterning the PMCs in vitro. Patterning is achieved by physically constraining PMCs, by creating adhesion contrast, or by a combination of these approaches. We use both soft-lithography and direct laser-milling to pattern surfaces.
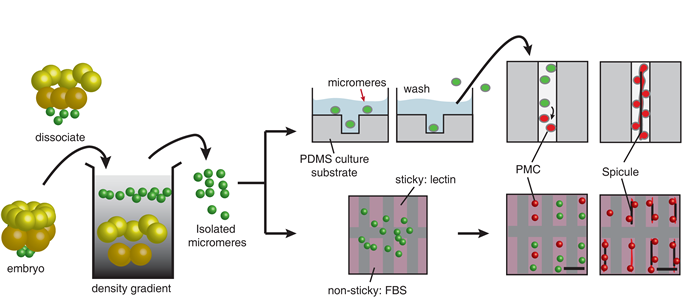
Figure 1. Work-flow of two conceptually different approaches for patterning the progenitor of the PMCs, the micromeres isolated from the early developmental stage of the sea urchin embryo in vitro.
Our preliminary results show the location of the PMCs and thus the growth direction of the spicules are greatly influenced by the guidance cues (Figure 2). Moreover, in a polarized light microscope, the spicules brighten/extinguish at the same time, indicating each spicule is a single crystalline calcite.
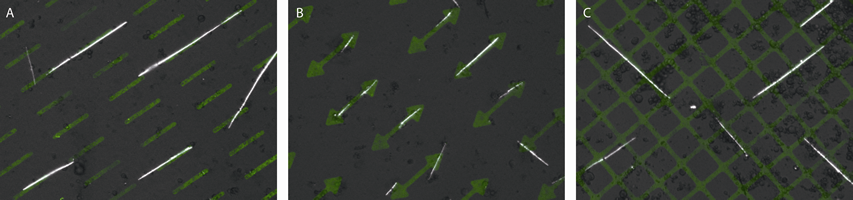
Figure 2. Spicules formed by the PMCs on the fluorescently labeled lectin Concanavalin A patterns in vitro. Each image comprises fluorescent light and polarized light micrographs. Note the growth direction (the long axis) of the spicules follows the pattern underneath.
Protein-Mediated Crystal Growth
Understanding the biological crystal growth machinery in vivo is key to understanding how to translate single crystal growth into an engineered process. Using STEM-HAADF, we observed the presence of a characteristic microstructure within the calcite matrix (figure 3). We suspect that the observed inclusions are proteins, which are distributed according to growth direction, following Kitajima and Urakami.
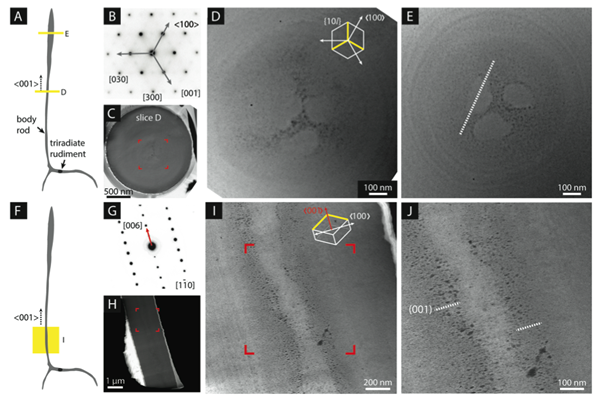
Figure 3. Inclusions of low atomic number or density appear dark against the background of the bright mineral matrix.
The microstructure for all growth directions is dominated by a series of concentric “growth rings”, as previously seen by Okazaki and Seto, while a newly observed, threefold symmetric “cloverleaf” feature runs parallel to the c-axis in the center of the body rod. We propose that the cloverleaf represents a growth sector boundary, caused by impurity incorporation at the edges of a faceted crystal. The cloverleaf is consistent with proteins (or other impurities) that show preferential binding to the leading {104} step edges during faceted growth. The occluded low-Z materials are pushed towards the edges of the {10l} faces by the advancing steps and are then occluded into the growing mineral. Concentric growth rings are indicative of low-Z materials stabilizing planes parallel to the growth-axis, resulting in the {hk0} faces that comprise the cylindrical body of the spicule. Periodic changes in the radial growth rate account for the distribution of growth rings. These two motifs suggest two growth mechanisms: faceted growth in the c-axis direction, and much slower growth in the radial direction.
Eventually, we hope the results from this study will not only provide understanding toward the cellular deposition machinery, but inspiration for developing novel "bottom-up" approaches to the synthesis of single crystals with the shape and structure desired for their specific application.
Home | Contact Us | Sites A-Z









