Minna Krejci, Brian Wasserman, Derk Joester
Collaborators: Stefan Vogt, Lydia Finney (X-ray Science Division, Argonne National Laboratory)
______________________________________________________________
Overview
The safe, selective removal of 90Sr from spent reactor fuels is critical for sustainable use of nuclear power, one of the few scalable and carbon-neutral technologies currently available for energy production. However, the chemical similarity of Ca2+, Sr2+, and Ba2+ cations presents a technological challenge for 90Sr separation. Incidentally, this same similarity leads to indiscriminate transport of these ions by most organisms. We are interested in the few organisms that do demonstrate selectivity for Ba2+ and/or Sr2+ over the much more abundant Ca2+ by selectively depositing highly unusual biominerals such as barite (BaSO4) and celestite (SrSO4). Specifically, we are studying the unicellular desmid green algae Closterium moniliferum, which precipitate BaSO4 crystals in small vacuoles at the tips of the cells (Figure 1).[1] Clearly, there is much to be learned from the strategies these organisms have evolved to address the selectivity problem, and we aim to understand the basic mechanisms in the hopes that they will provide inspiration for the next generation of materials for 90Sr selective separation.
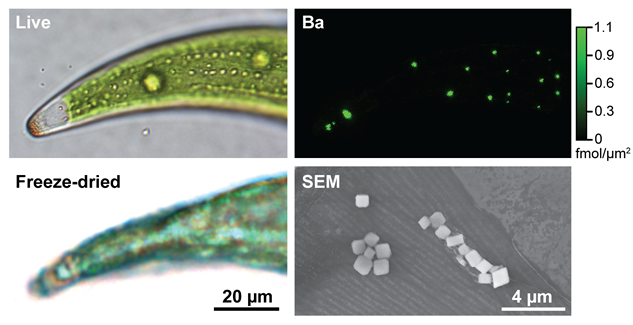
Figure 1. BaSO4 crystals in desmid green algae. One half of a crescent-shaped live cell is shown (top left), as well as a comparable cell after preparation for synchrotron X-ray fluorescence (SXRF) microscopy by plunge-freezing and freeze-drying (bottom left). A Ba SXRF map (top right) of this freeze-dried cell shows Ba hot spots corresponding to BaSO4 crystals. The morphology of these crystals can be seen in an SEM image of a cell that has been ashed (bottom right).
Synchrotron X-ray Fluorescence Microscopy
The mechanisms of selectivity in Sr/Ba-mineralizing organisms have remained largely unexplored, due in part to a lack of imaging techniques that could allow for simultaneous and selective visualization of subcellular distributions of the elements of interest (i.e., Ba, Sr and Ca). However, this is now possible due to recent developments in synchrotron X-ray fluorescence (SXRF) microscopy at third generation sources, which offers extremely low minimum detection limits (>10-20 mol/µm2)[2] and subcellular resolution (>150 nm),[3] without the need for exogenous probes. Unlike optical microscopy, it can be used to quantify multiple, even chemically similar elements at the same time, independent of whether they are dissolved, bound, or in crystalline form. A growing number of studies have taken advantage of these unprecedented capabilities, for example to quantify and visualize platinum cancer drugs,[4] magnetic resonance contrast agents,[5] and endogenous metals during angiogenesis and macrophage differentiation.[6, 7]
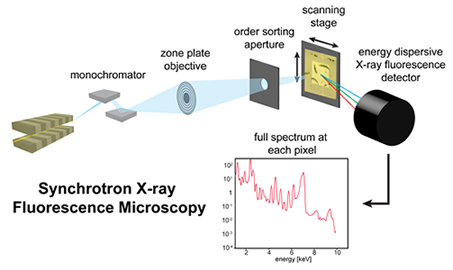
X-ray fluorescence occurs when a hard X-ray hitting the sample is photoelectrically absorbed, resulting in the ejection of a core-shell electron. A higher-shell electron falls to fill the vacancy, and a photon is emitted with an energy equal to the difference between the two shells.[3] This energy is element-specific. In SXRF microscopy, X-ray optics are used to focus incident hard X-rays from a synchrotron source onto a small spot on the sample (from several nanometers to several microns in diameter). The sample is raster-scanned through the beam, and an energy-dispersive detector is used to acquire a full fluorescence spectrum, with peaks corresponding to specific elements, at each pixel. By comparing these spectra to reference standards of known concentration, quantitative elemental maps of multiple elements (~10 or more) can be created simultaneously.
Selective Transport or Selective Precipitation?
Because SXRF microscopy gives us the ability to visualize the intracellular dynamics of Ca, Sr, Ba, and S (among other elements) simultaneously, we are using this technique to unravel the mechanisms involved in the unusual selectivity exhibited by C. moniliferum. We have verified and quantified the uptake and sequestration of Ba in BaSO4 crystals (Figure 2). In cells exposed to Sr-supplemented culture medium, we observe time‐dependent changes in intracellular Sr con-centrations, as well as Sr substitution for Ba in crystals. The kinetics of uptake and efflux of Sr appear to be dependent on external Ca concentrations, and Sr, Ba, and Ca show similar cellular localization. A highly selective cross-membrane transport step is not evident. Instead, high S levels detected in the vacuole (Figure 3) may indicate that selectivity occurs via selective precipitation of low solubility compounds. In this "sulfate trap" model, high sulfate levels and the presence of soluble Ba in the vacuole leads to preferential precipitation of BaSO4 and (Ba,Sr)SO4 due to their low solubilities relative to CaSO4.
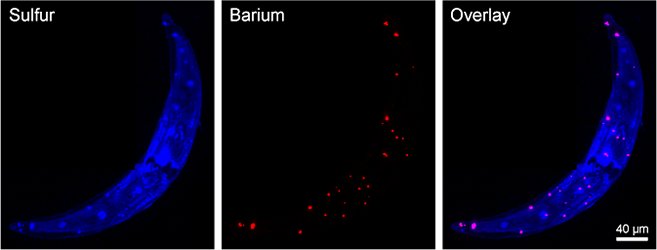
Figure 2. Ba and S hot spots in SXRF maps of C. moniliferum correspond to BaSO4 crystals, which can be found in the terminal vacuoles and throughout the cell.
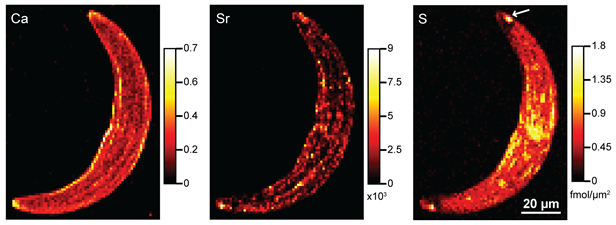
Figure 3. SXRF maps of Ca, Sr, and S in a C. moniliferum cell that has been exposed to Sr. Ca and Sr show similar distributions, and high S concentrations in the terminal vacuoles at the tips of the cell (arrow) may indicate a sulfate trap or selective precipitation mechanism of selectivity.
Based on this model, we show that it is possible to engineer desmid growth conditions to increase Sr incorporation into the biomineral by several orders of magnitude. This introduces the promising possibility of using desmids for phytoremediation of 90Sr environmental contamination. The birds-eye view of intracellular Ba/Sr ion trafficking presented by SXRF elemental mapping allows for the identification of areas for further investigation that may ultimately provide insight for the development of bio-inspired materials for 90Sr separation.
References
[1] J. Wilcock, C. Perry, R. Williams, A. Brook, Proceedings of the Royal Society of London, Series B: Biological Sciences 1989, 203.
[2] B. S. Twining, S. B. Baines, N. S. Fisher, J. Maser, S. Vogt, C. Jacobsen, A. Tovar-Sanchez, S. A. Sanudo-Wilhelmy, Analytical Chemistry 2003, 75, 3806.
[3] C. Fahrni, Current opinion in chemical biology 2007, 11, 121.
[4] M. D. Hall, R. A. Alderden, M. Zhang, P. J. Beale, Z. H. Cai, B. Lai, A. P. J. Stampfl, T. W. Hambley, Journal of Structural Biology 2006, 155, 38.
[5] P. J. Endres, K. W. MacRenaris, S. Vogt, M. J. Allen, T. J. Meade, Molecular Imaging 2006, 5, 485.
[6] L. Finney, S. Mandava, L. Ursos, W. Zhang, D. Rodi, S. Vogt, D. Legnini, J. Maser, F. Ikpatt, O. I. Olopade, D. Glesne, Proceedings of the National Academy of Sciences of the United States of America 2007, 104, 2247.
[7] D. Glesne, S. Vogt, J. Maser, D. Legnini, E. Huberman, Journal of Structural Biology 2006, 155, 2.
Home | Contact Us | Sites A-Z









