The Joester group uses state of the art methods in materials science and engineering to elucidate the properties and formation mechanisms of biominerals. These complex tissues are made by organisms under physiological conditions with exquisite control over mineral composition and morphology. Currently active projects in our group include:
Bioengineering Single Crystal Growth
Sr Mineralization in Acantharia
Nucleation from Amorphous Precursors
Formation of High-Magnesium Calcite in Sea Urchin Teeth
The Enamel Atlas
Enamel is a principal component of the dentition (Figure 1). Defects in this hierarchically structured hard tissue are associated with a wide variety of diseases and constitute an important public health problem.EA1 Conditions range from the relatively rare amelogenesis imperfecta (AI), to those that affect a large percentage of the population, such as molar incisor hypo-mineralization (MIH) and caries (tooth decay). They share a tremendous impact on the quality of life and together contribute to exorbitant cost of dental care, greater than $150b/year in the US alone.
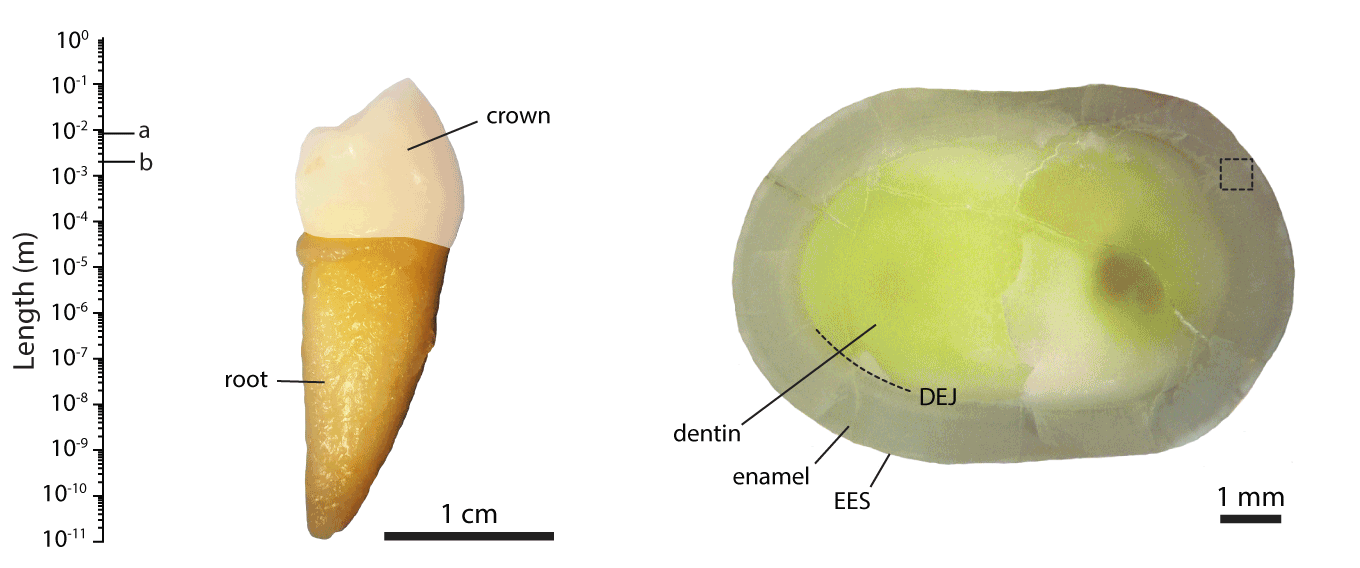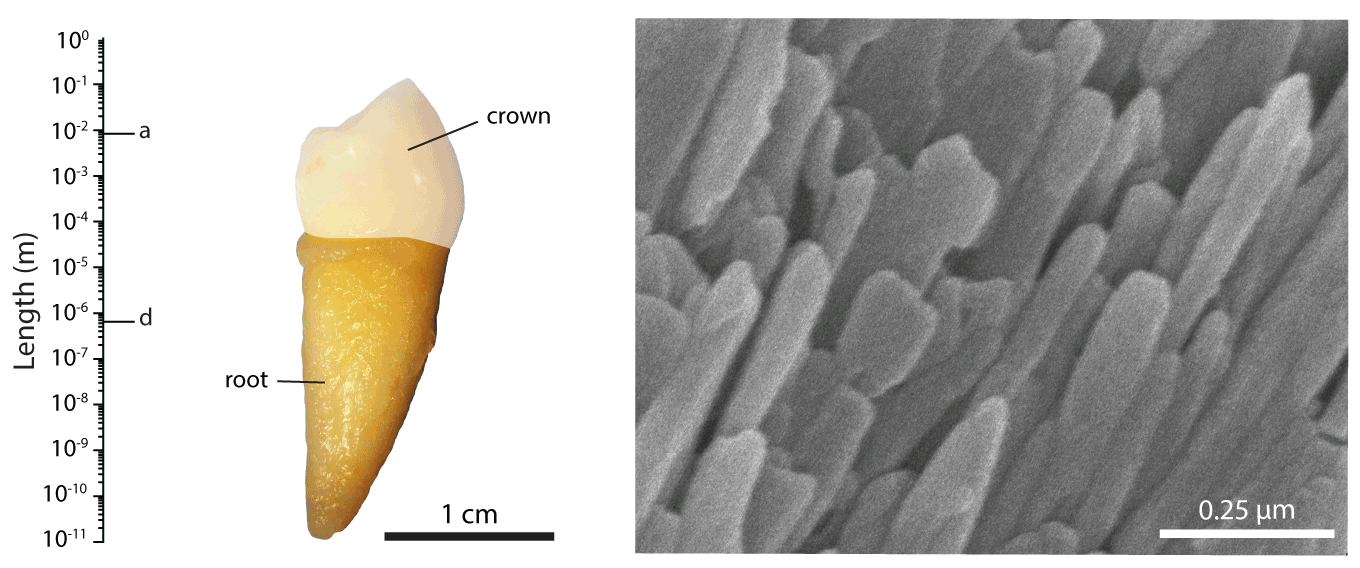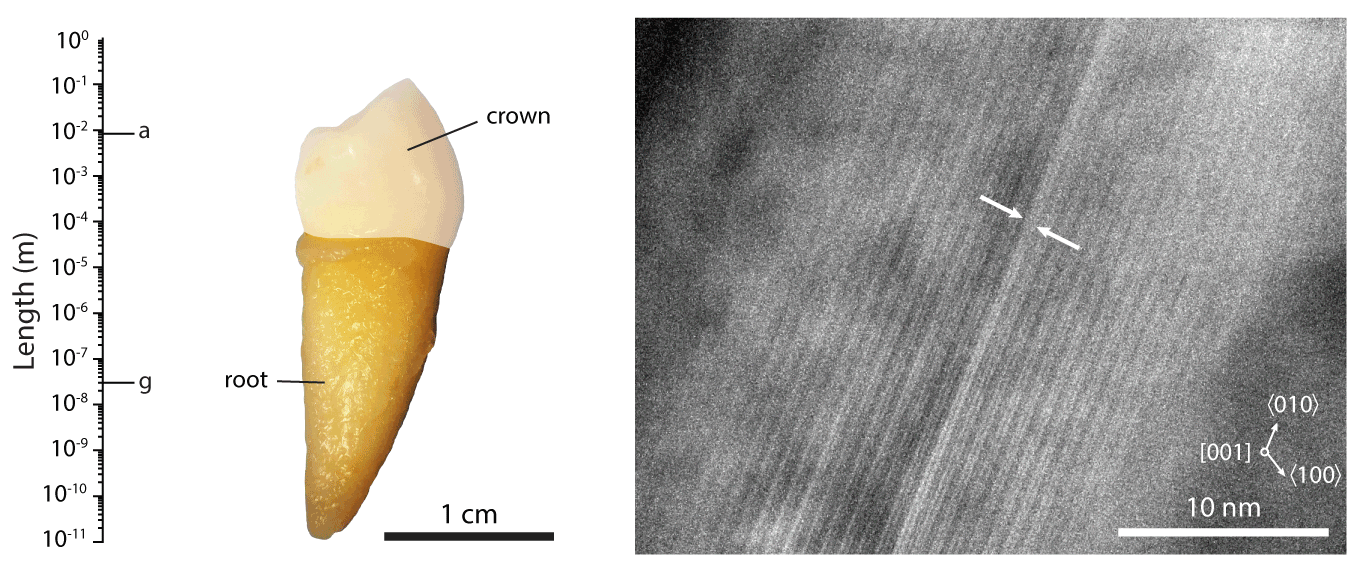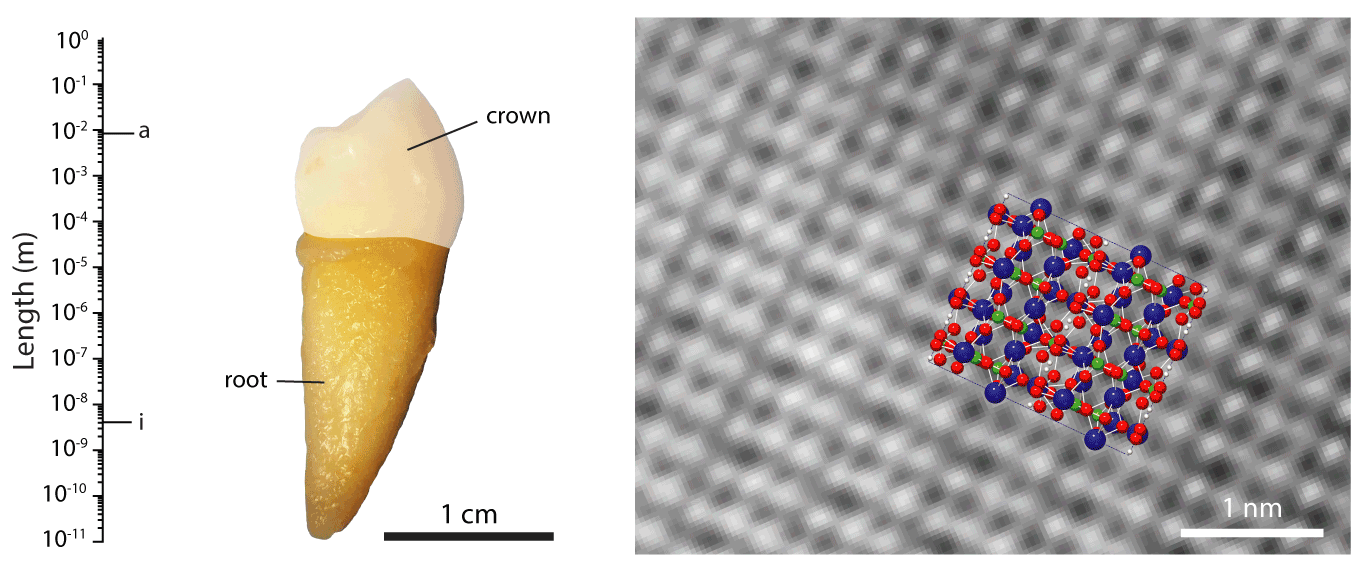
Figure 1.The hierarchical length scales of human dental enamel, adapted from ref []
Even though significant progress has been made in our understanding of the genetic, physiological and developmental mechanisms that drive the secretion, maturation, and eventual degradation of enamel, many crucial questions remain unanswered, including the nature of the cellular processes, protein-protein and protein-mineral interactions involved, how the underlying biology determines the biophysical and structural properties of the mineralized tissue, and how structural and compositional hierarchies control the kinetics of lesion formation.
We are working with collaborators around the world to address these issues, contributing our expertise in structural and compositional analyses of mineralized tissues. For instance, we pioneered the use of atom probe tomography (APT, see also the Northwestern University Center for Atom Probe Tomography), a chemical imaging technique with unrivaled spatial resolution and chemical sensitivity. We discovered the presence of an amorphous intergranular film that glues together enamel crystallites,EA2-EA3 and found that in human enamel, crystallites have a core-shell structure (Figure 2).EA4 We furthermore use synchrotron micro-computed tomography (SMCT) and micro-diffraction (µXRD), and electron optical methods (STEM, STEM-EDS, STEM-EELS), among others.
Figure 2. Core-shell and sandwich structure of human enamel crystallite.Atom probe tomography reconstruction of human enamel, 1nm voxel size. Colors indicate concentration iso-surfaces for 24Mg2+ (magenta, c = 0.1nm-3), 23Na+ (turqouise, 0.3nm-3), COxHy+ (red, 0.12nm-3), and and 40Ca19F+ (blue, 0.07nm-3). Visualization generated using Matlab R2019b. Adapted from ref []
Current projects:
- Using APT, SMCT, µXRD, and correlative techniques we work to improve our understanding of the progression of enamel nanostructure and phase composition as caries lesions develop (Figure 3).EA5-EA6 Prof. Stuart Stock is a frequent collaborator when using SCMT and µXRD.
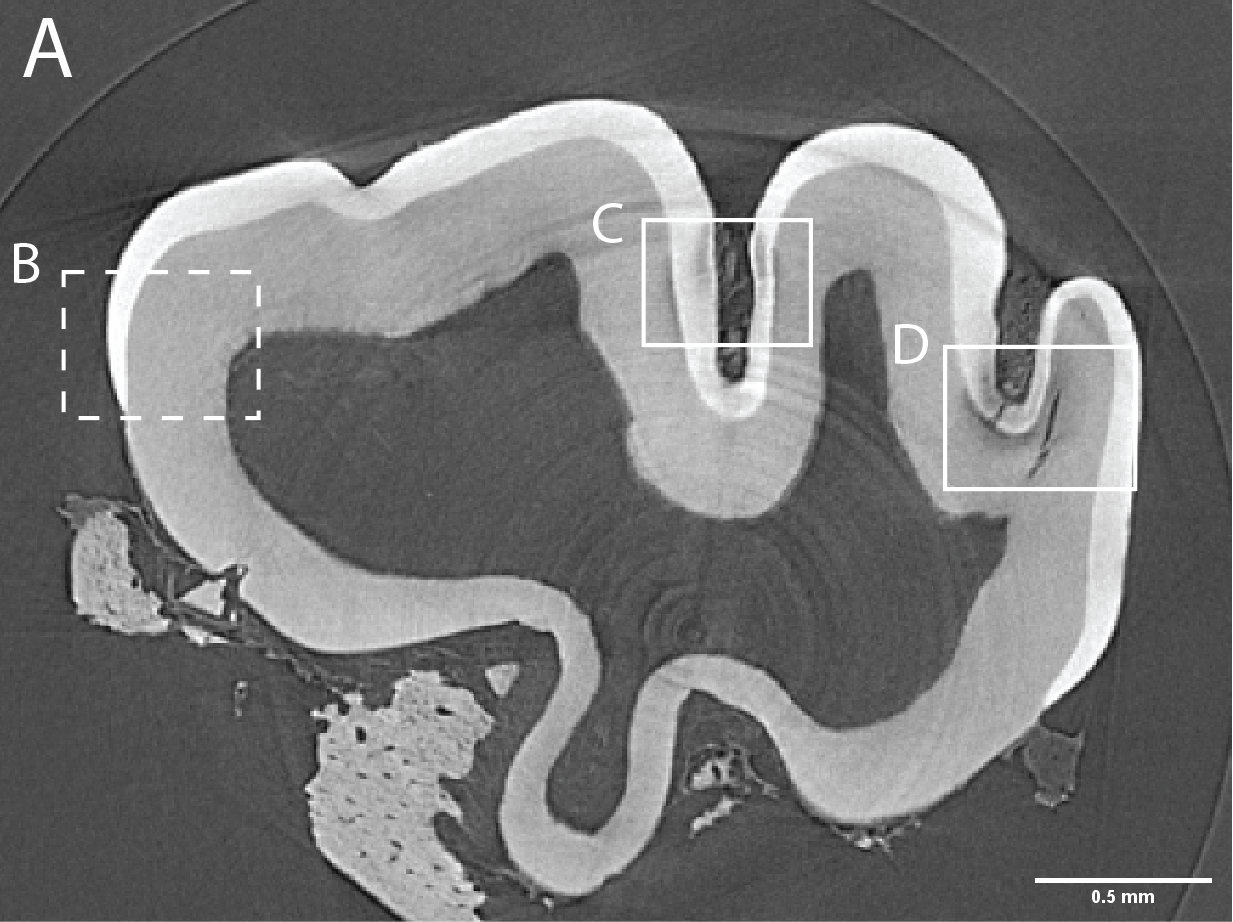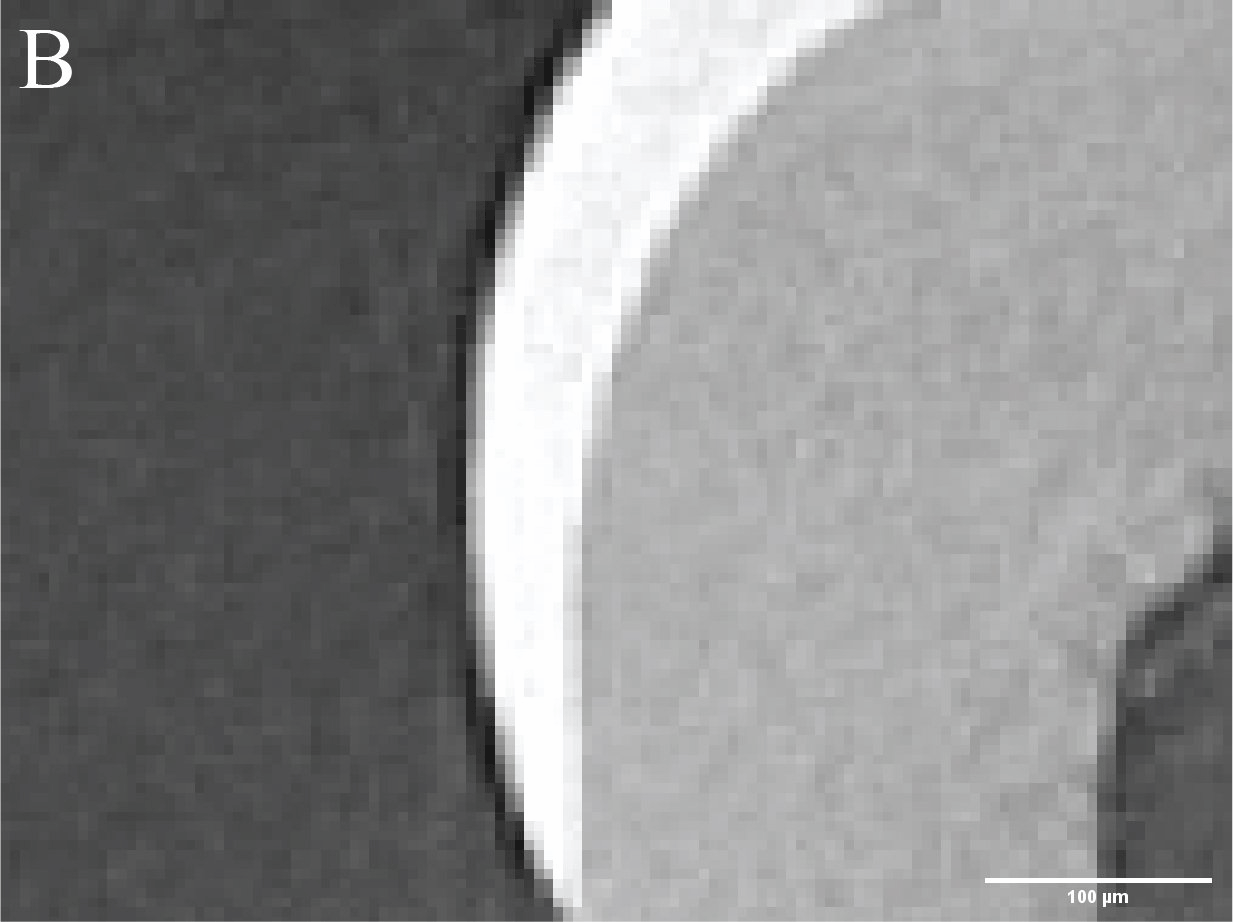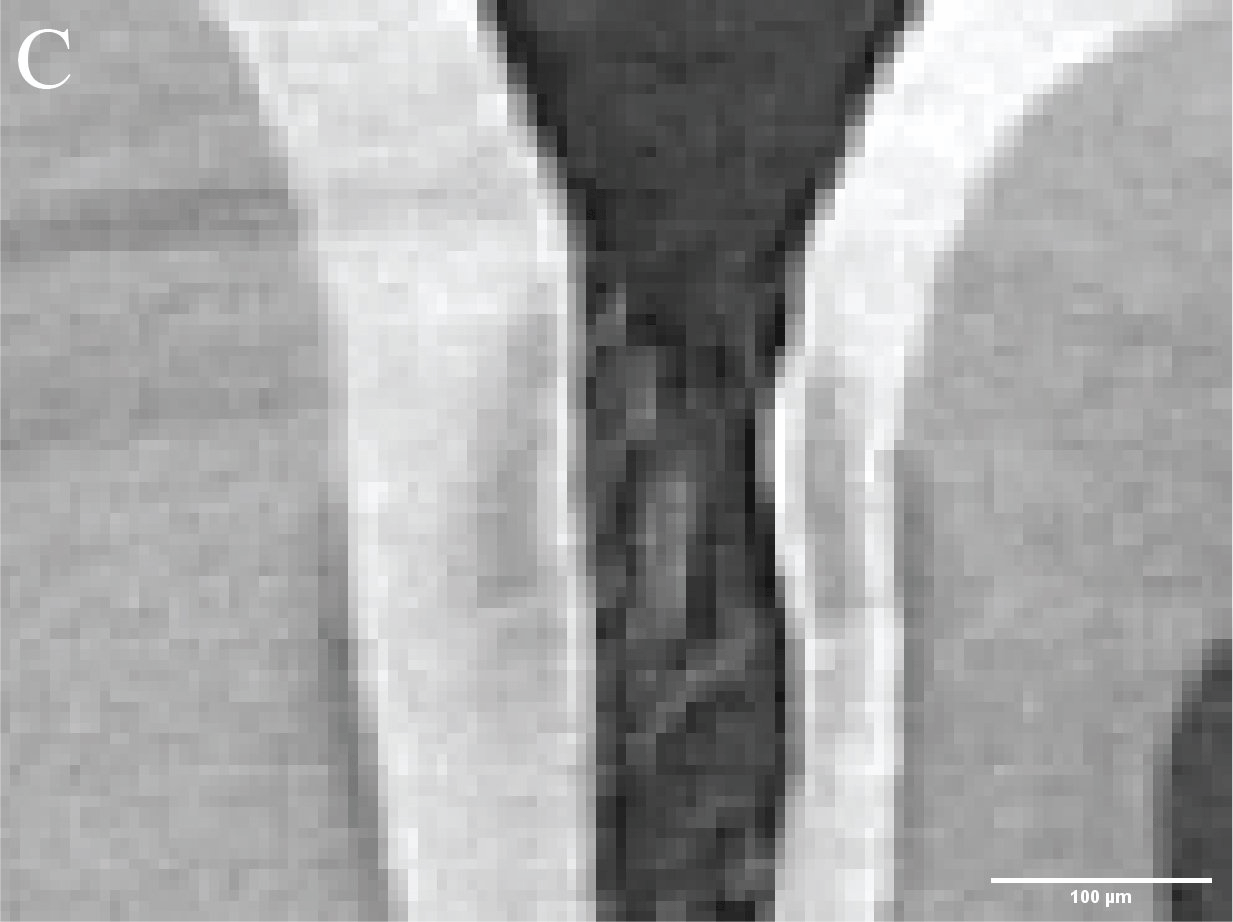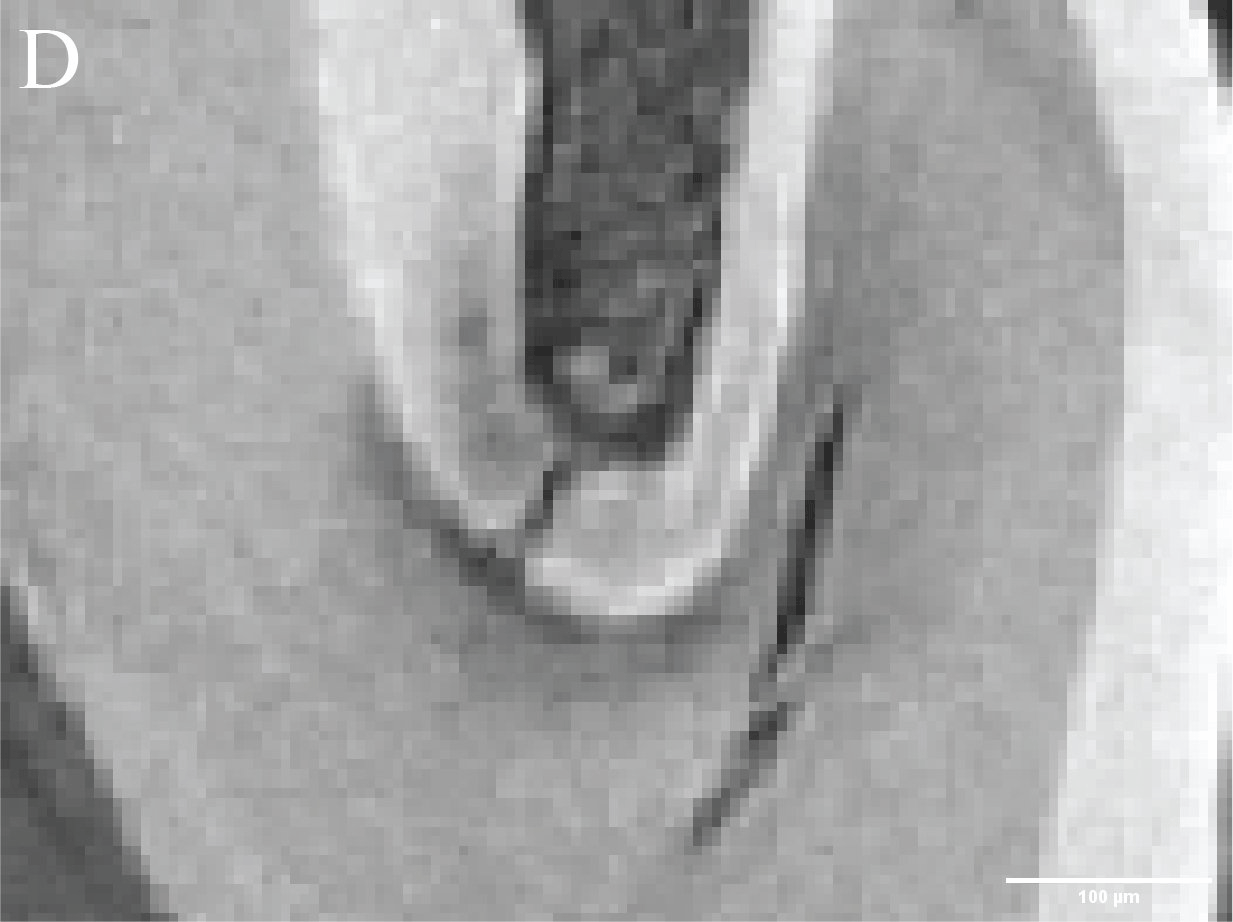
Figure 3. Caries lesions in rat molars. A) Slice through SMCT reconstruction of M1 rat molar. B-D: Close up of healthy enamel (B) and caries lesions (C,D). - Using a broad range of high throughput and high-resolution techniques, and machine learning approaches (Figure 4), we are creating the biophysical EnamelAtlas that will enable quantitative comparison of mature and forming enamel in wildtype mice and a large number of genetically engineered mutants produced by Profs. Ophir Klein and Jeffrey Bush (UCSF), Prof. Michael Paine (USC), Prof. Tom Diekwisch (TAMU), and Profs. Jan Hu and Jim Simmer (University of Michigan).
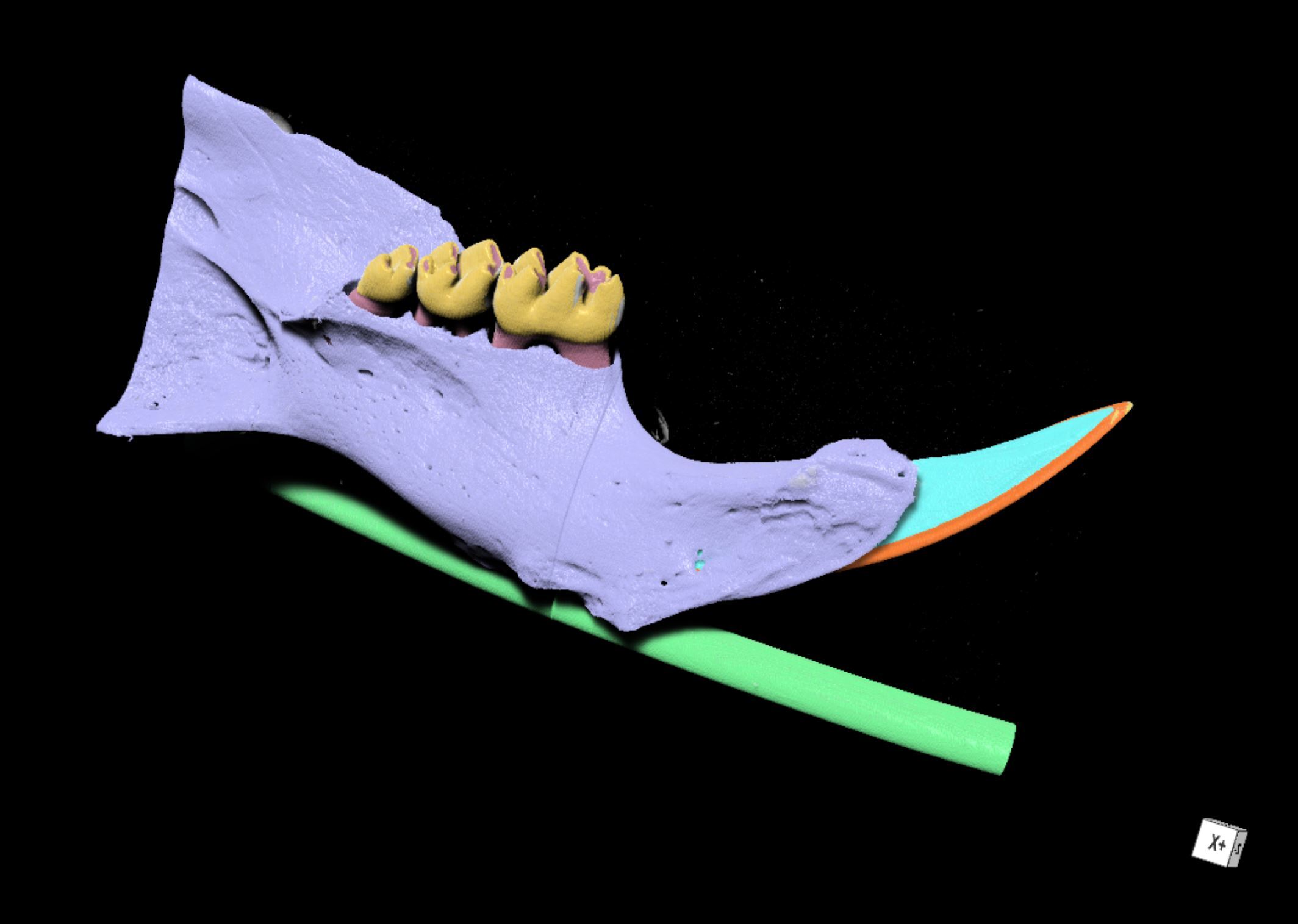
Figure 4. Automated segmentation using a convolutional neural network (CNN). Rendering of segmented SMCT reconstruction of a wildtype mouse mandible with bone (purple), molar enamel (yellow), incisor enamel (orange), and dentin (cyan) segments highlighted. - Together with Profs. Peter Voorhees and James Rondinelli (Northwestern University) we build descriptive and predictive atomistic (DFT, AIMD) and continuum (phase field) models of the progression of enamel nanostructure as caries lesions develop from the nanometer to micrometer scales, utilizing high-fidelity experimental data combined with state-of-the-art materials simulations techniques (Figure 5).
- Together with Prof. Michael Koo (UPenn), we are exploring the mechanism of action of multi-functional nanoparticles designed to combat both bacterial biofilms and strengthen enamel.
- Together with Prof. Ana Bedran-Russo (Marquette University), we study the impact of aging on dentin.
- Together with Dr. Sophia Houari-Meiri (), we characterize the structure and composition of human teeth affected by fluorosis and MIH.
- Together with Dr. Felicitas Bidlack (Forsyth Institute), we study the role of enamel matrix proteins in enamel development.
- Finally, we are part of the Developmental Dental Defects Group (D3G), a global translational research and education network spearheaded by Prof. Mike Hubbard.
We anticipate that the knowledge generated by in these experimental and computational projects will:
- clarify the mechanism of de- and remineralization that underlie caries lesion formation.
- clarify fundemental mechanisms of enamel development and its pathologies.
- identify biological strategies to harden enamel against acid corrosion.
- provide information integral to developing early caries detection schemes.
- aid in the development of new materials for minimally invasive caries prevention to supersede current surgical management of tooth decay.
BACK TO TOP
Bioengineering Single Crystal Growth (NSF)
Mineralized tissues are sophisticated hybrid materials with highly hierarchical architecture. Despite great recent progress in bio-inspired materials synthesis, many hallmarks of biological crystal growth have yet to be reproduced in vitro. Clearly, much could be gained by developing a biotechnological alternative to materials synthesis. Sea urchin embryo biomineralization exemplifies this unrivaled level of biological control over the inorganic mineral: smoothly curving, branching, yet single crystalline spicules are created via transient amorphous precursors (Figure 1).[1-7]
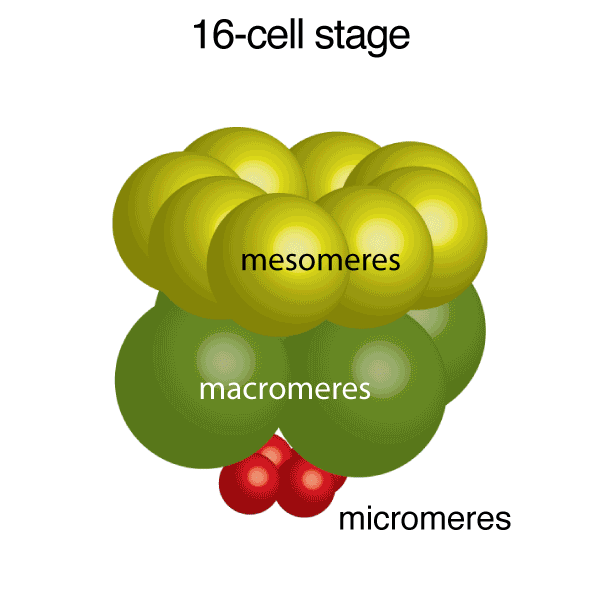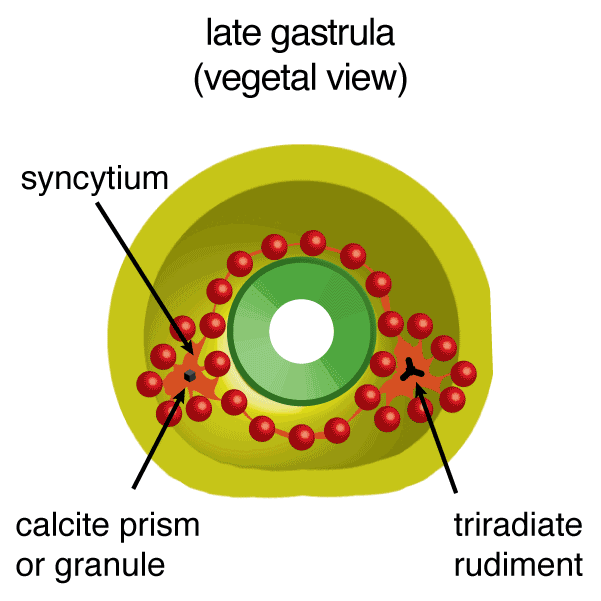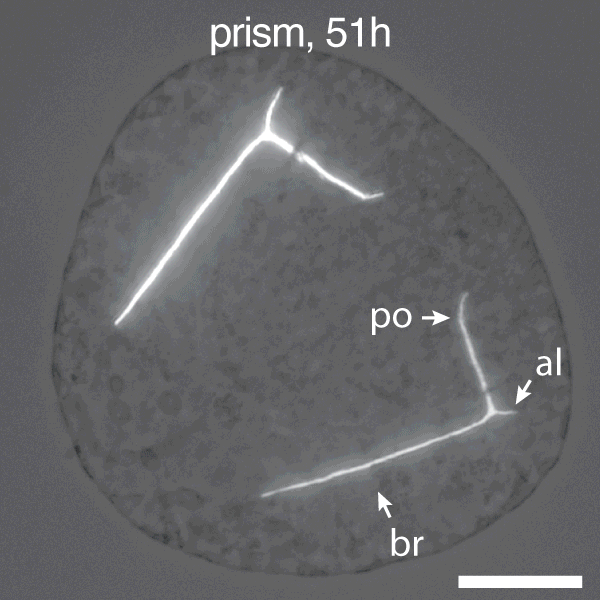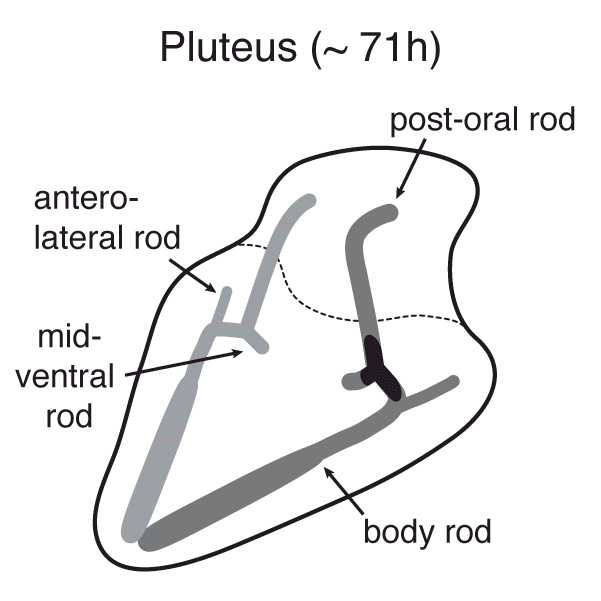
Figure 1. Development of Strongylocentrotus purpuratus embryos and formation of endoskeleton comprised of two single crystalline calcite spicules by primary mesenchyme cells (PMCs).
What makes the sea urchin system truly unique, however, is that primary mesenchyme cells (PMCs) that form the skeletogenic organ retain this ability in cell culture.8-9 This is a highly desirable, if exceedingly rare, feature for engineered tissues. It is key to our long-term goal of mastering the capabilities of PMCs for biotechnological material syntheses.
Towards this goal, and building on the pioneering work of Okazaki and Wilt (reviewed in 10), we have developed tools to control the deposition of single crystalline calcite (CaCO3) spicules in vitro.11 We further discovered that vascular endothelial growth factor (VEGF) signaling controls the shape and crystallographic growth direction of spicules deposited by PMC. VEGF does so without directly interacting with the mineral (Figure 2).12
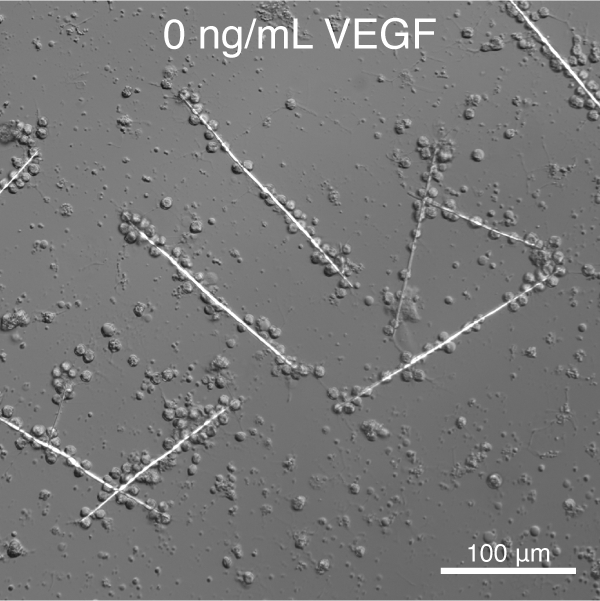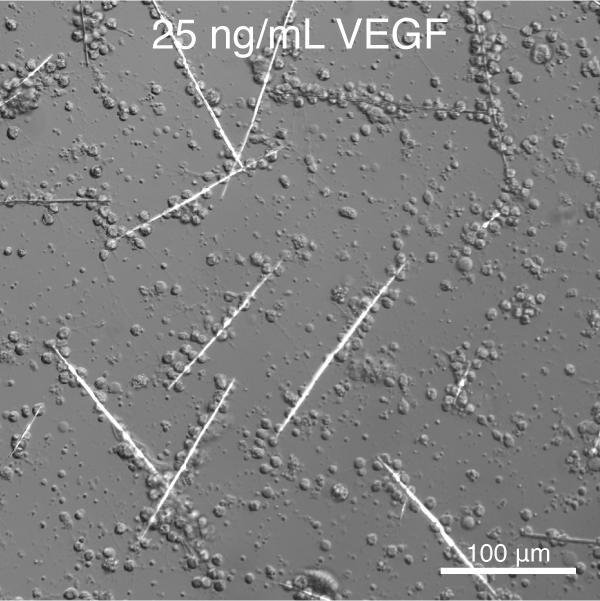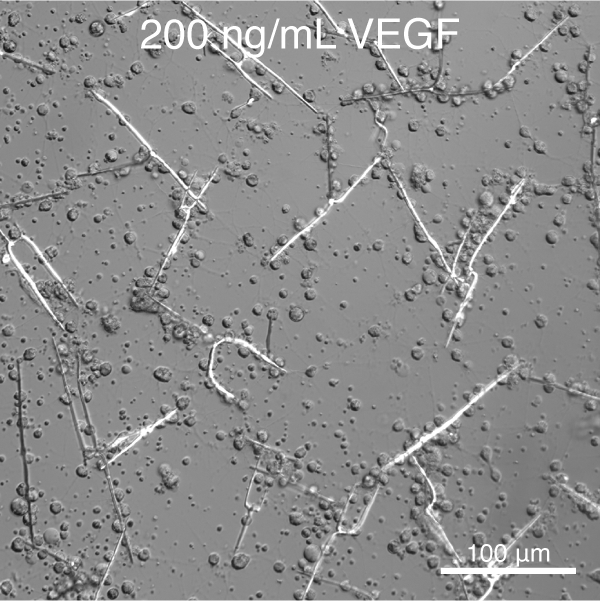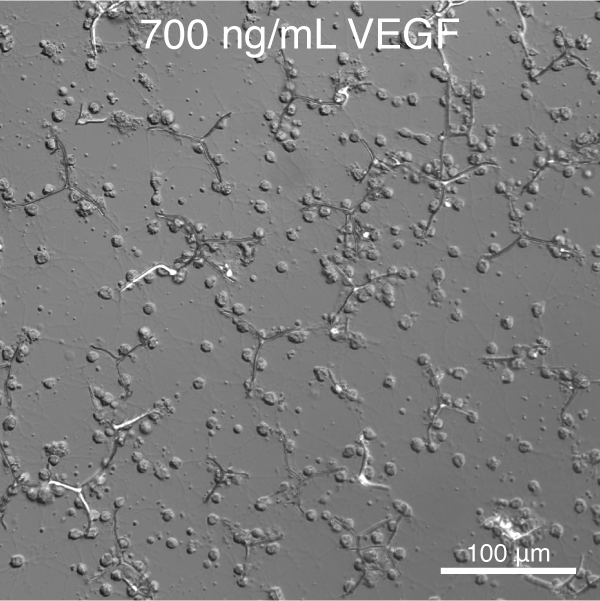
Figure 1. S. purpuratus PMCs cultured in the presence of recombinant SpVEGF deposit linear spicules at low concentration and triradiate spicules at high concentration.
Recently, transcriptional profiling using RNA-seq resulted in deep additional insights into potential molecular players involved in this process. This provides the unique opportunity to investigate the mechanism underlying this remarkable example of biological control over crystal growth in a well-characterized in vitro cell culture system.
Current projects:
- Determine the distribution of different spicule matrix proteins in the single crystalline matrix using an approach pioneered by Kitajima and coworkers (Figure 3).13
- Characterize the role of spicule matrix proteins in controlling nucleation and growth using a microfluidic assay.
- Determine the role of the cytoskeleton and cytoskeleton-dependent processes in spicule growth and branching (Figure 4).
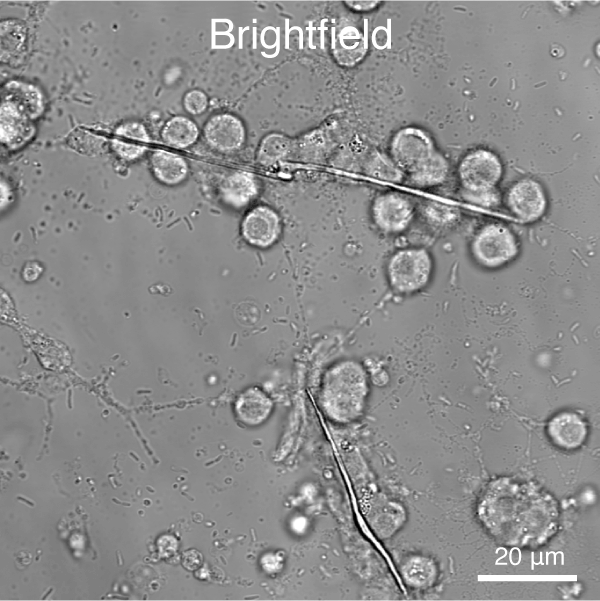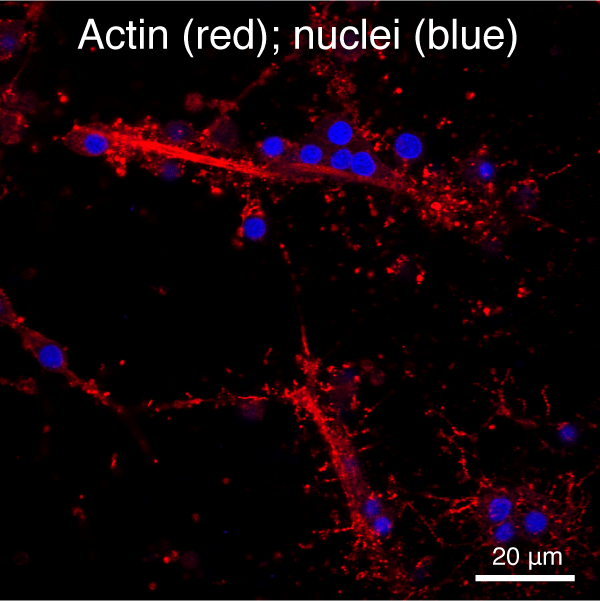
Figure 4. Automated segmentation using a convolutional neural network (CNN). S. purpuratus PMCs in cell culture stained for filamentous actin (red) and nuclei (blue). - Analyze cell behavior and ultrastructure of PMCs in response to VEGF treatment (Figure 5)
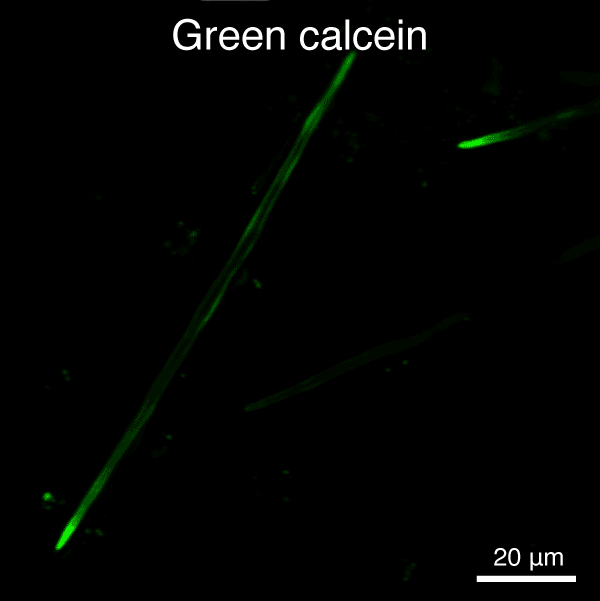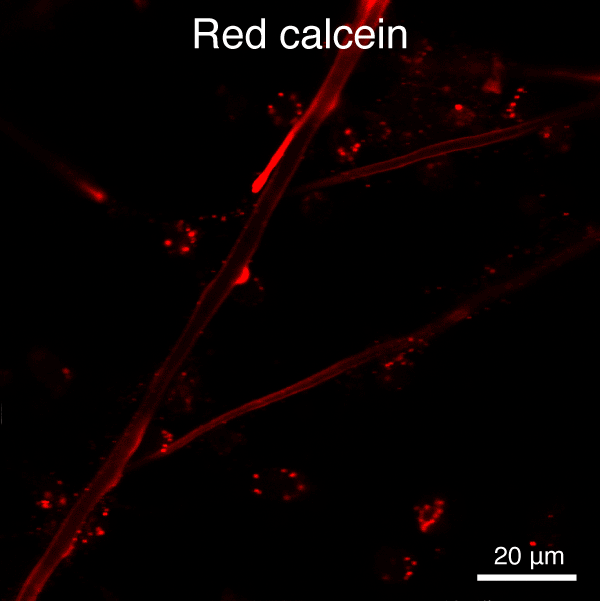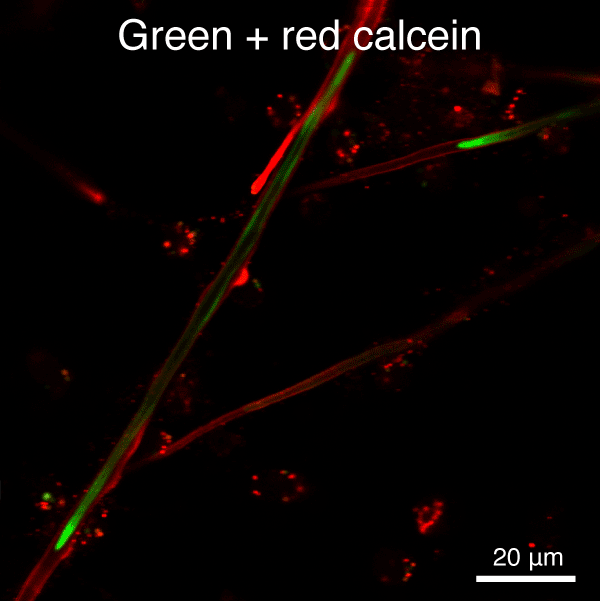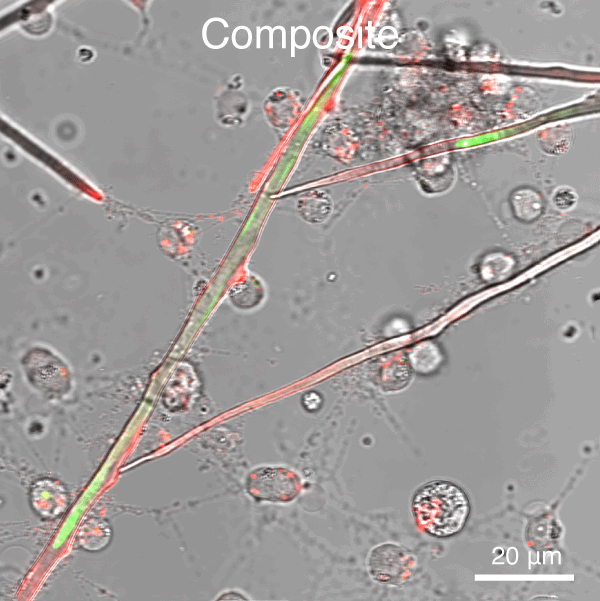
Figure 5. Automated segmentation using a convolutional neural network (CNN). S. purpuratus PMCs in culture labeled with dyes that are endocytosed, redistributed in vesicles, and ultimately label the spicule mineral. Here, two consecutive pulse-chase labeling experiments were performed using different color dyes.
References:
- Y. Politi, Y. Levi-Kalisman, S. Raz, F. Wilt, L. Addadi, S. Weiner, I. Sagi, Adv. Funct. Mater. 2006, 16, 1289-1298. “Structural characterization of the transient amorphous calcium carbonate precursor phase in sea urchin embryos”.
- Y. Politi, T. Arad, E. Klein, S. Weiner, L. Addadi, Science 2004, 306, 1161-1164. “Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase”.
- S. Weiner, Y. Levi-Kalisman, S. Raz, L. Addadi, Connect. Tissue Res. 2003, 44, 214-218. “Biologically formed amorphous calcium carbonate”.
- S. Raz, P. C. Hamilton, F. H. Wilt, S. Weiner, L. Addadi, Adv. Funct. Mater. 2003, 13, 480-486. “The transient phase of amorphous calcium carbonate in sea urchin larval spicules: The involvement of proteins and magnesium ions in its formation and stabilization”.
- L. Addadi, S. Raz, S. Weiner, Adv. Mater. 2003, 15, 959-970. “Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization”.
- E. Beniash, L. Addadi, S. Weiner, J Struct Biol 1999, 125, 50-62. “Cellular Control over Spicule Formation in Sea Urchin Embryos: A Structural Apporach”.
- E. Beniash, J. Aizenberg, L. Addadi, S. Weiner, Proceedings of the Royal Society of London Series B-Biological Sciences 1997, 264, 461-465. “Amorphous calcium carbonate transforms into calcite during sea urchin larval spicule growth”.
- K. Okazaki, Am Zool 1975, 15, 567-581. “Spicule Formation by Isolated Micromeres of Sea-Urchin Embryo”.
- T. Kitajima, K. Okazaki, Dev Growth Differ 1980, 22, 265-279. “Spicule Formation Invitro by the Descendants of Precocious Micromere Formed at the 8-Cell Stage of Sea-Urchin Embryo”.
- B. Moreno, A. DiCorato, A. Park, K. Mobilia, R. Knapp, R. Bleher, C. Wilke, K. Alvares, D. Joester, Methods Cell Biol 2019, 150, 293-330. “Culture of and experiments with sea urchin embryo primary mesenchyme cells.”. 10.1016/bs.mcb.2019.01.002.
- C.-H. Wu, R. T. Knapp, C. C. Tester, W. Gu, D. Jang, J. Greer, B. Lai, S. Chen, C. Sun, M. Balasubramanian, D. Joester, Microsc Microanal 2013, 19, 1632-1633. “Buried Interfaces and Phase Transformations in a Biogenic Single Crystal”. 10.1017/S1431927613010155.
- R. T. Knapp, C.-H. Wu, K. C. Mobilia, D. Joester, J Am Chem Soc 2012, 134, 17908-17911. “Recombinant Sea Urchin Vascular Endothelial Growth Factor Directs Single-Crystal Growth and Branching in Vitro”. 10.1021/ja309024b.
- T. Kitajima, H. Urakami, Dev Growth Differ 2000, 42, 295-306. “Differential distribution of spicule matrix proteins in the sea urchin embryo skeleton”.
Sr Mineralization in Acantharia
Acantharea are marine, unicellular organisms that produce endoskeletal spicules comprised of celestine (SrSO4), a highly unusual biomineral. This capability is remarkable not only because of the high level of biological control over single-crystal growth evident in species-specific morphological features (Figure 7), but also because of the high selectivity for strontium ions. In most organisms, including humans, the chemical similarity of Ca2+, Sr2+, and Ba2+ leads to rather indiscriminate transport. Therefore, 90Sr is readily taken up and incorporated into mineralized tissues such as bone, where its radioactive decay is a long-term cancer hazard. Yet, Acantharea sequester Sr2+ from seawater, where Ca2+ is present in more than 100-fold excess. Clearly, Acantharea have evolved to manipulate Sr ions and SrSO4 far beyond human capabilities. Understanding the mechanism by which Acantharea selectively sequester Sr2+ could transform our ability to remove 90Sr from high level radioactive waste such as spent nuclear fuel rods, or the environment after accidental release (e.g. Fukushima incident).
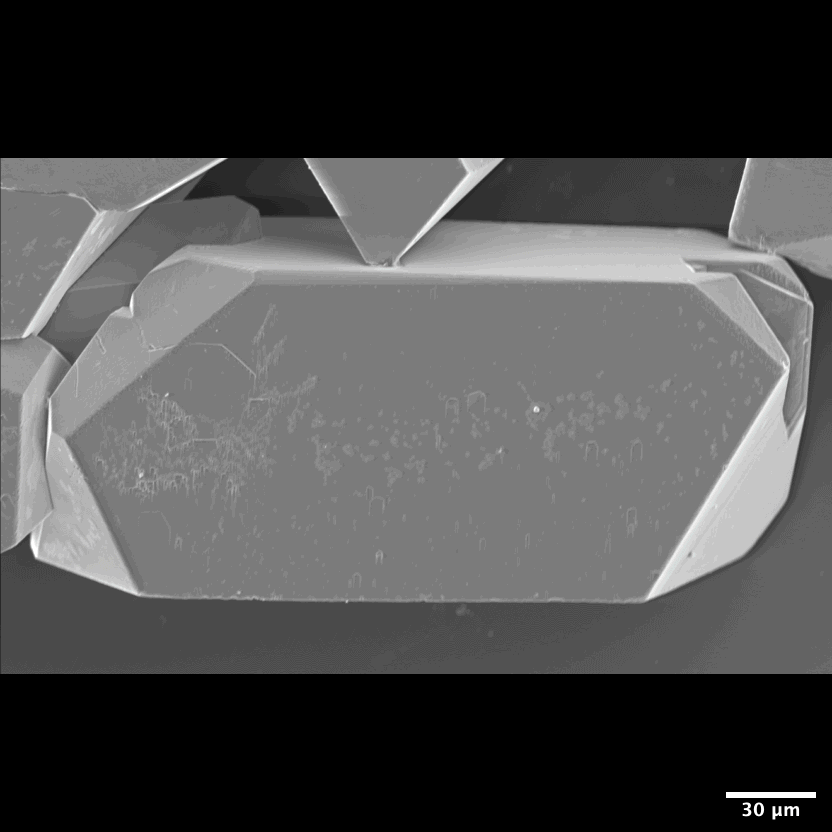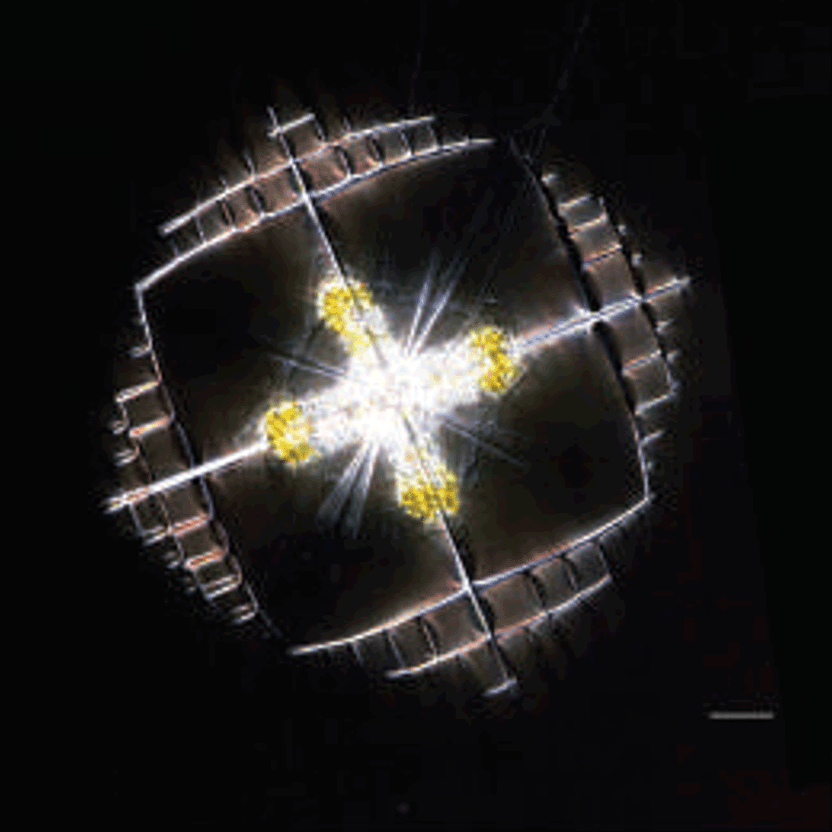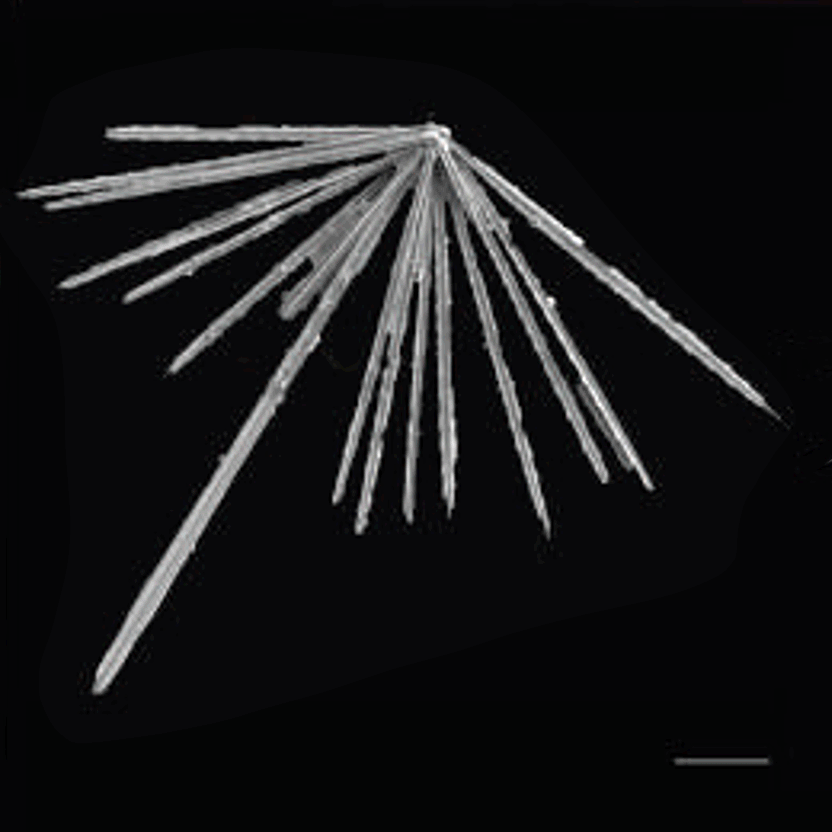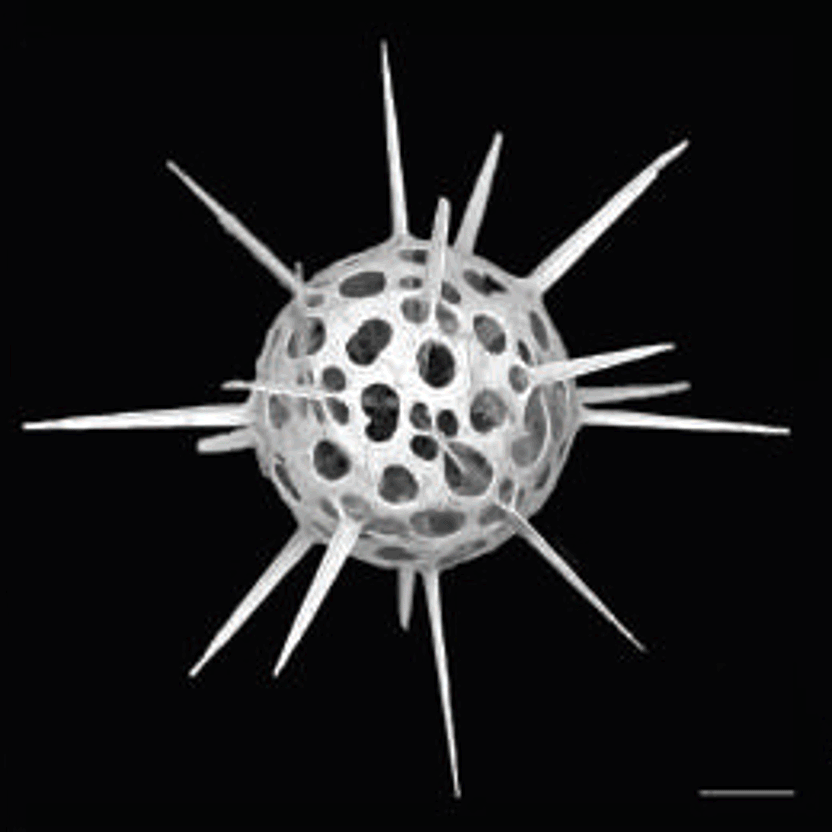
Figure 7: Habit of celestine (SrSO4) single crystals Light microscope and scanning electron microscope images of synthetic celestine single crystals and single-crystalline endoskeletal spicules of several Acantharea species. Acantharea images adapted from SR1.
Despite their exceptional features, Acantharea biomineralization remains largely unexplored. This is to a large degree because they were difficult to collect and preserve, and because there are no genomic resources available. In this project, we investigate the molecular mechanisms by which Acantharea control morphogenesis and composition of their endoskeletal spicules. Towards this goal, we use a broad range of sophisticated materials characterization techniques, including scanning transmission electron microscopy (STEM), X-ray fluorescence microscopy (XFM), nano-X-ray diffraction (nano-XRD), and atom probe tomography (APT), as well as vibrational spectro-microscopy to contrast and compare the nanoscale structure and composition of spicules from different species. We furthermore use -omics approaches to profile genes and proteins involved in this process. We could not do this without collaborators around the world who have helped us collect specimens: Prof. Dave Carron (USC), Dr. John Burns (Bigelow), Dr. Johann Decelle (ESRF), …
BACK TO TOPNucleation and Growth in Confinement
Phase transformations of carbonates are relevant to a wide range of biological, environmental, and industrial processes. Over the past decade, it emerged that crystallization pathways in these systems can be quite complex (Figure 8).NG1 Metastable intermediates, such as amorphous calcium carbonate (ACC), greatly impact composition, structure, and properties of more stable phases. Especially in biological systems, the role of confinement, for instance in intracellular vesicles, and of soluble, and insoluble additives remain poorly understood.NG2 This is in part due to the rapid transformation of ACC in bulk systems.
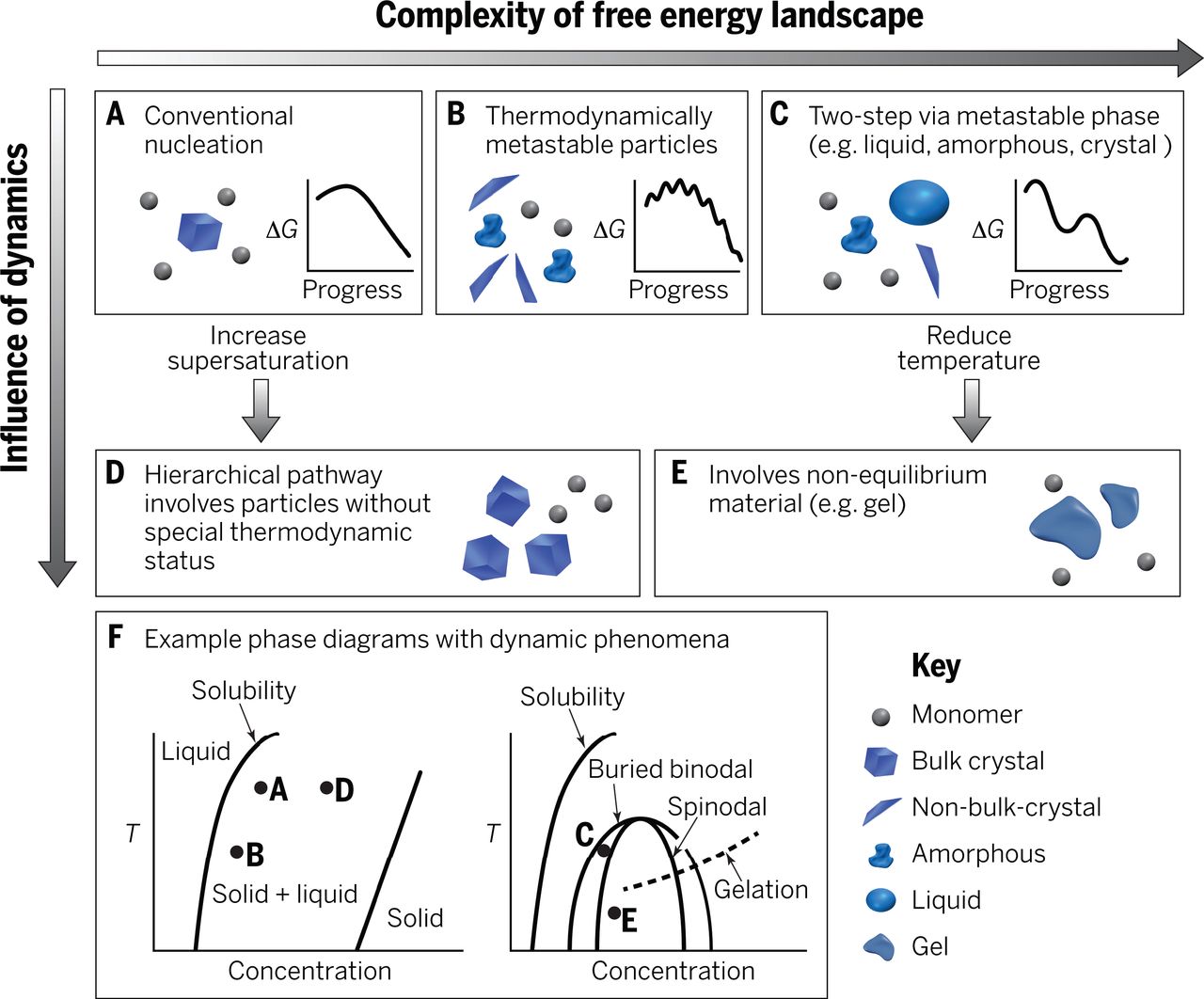
Figure 8: Crystallization pathways The possible pathways by which monomers form a stable bulk crystal, and the physical mechanisms that give rise to them. Adapted from Ref.1
We have developed an assay using microfluidic droplet reactors to determine nucleation rates (Figure 9NG3).NG3-NG4
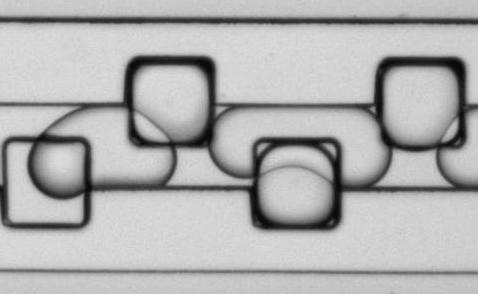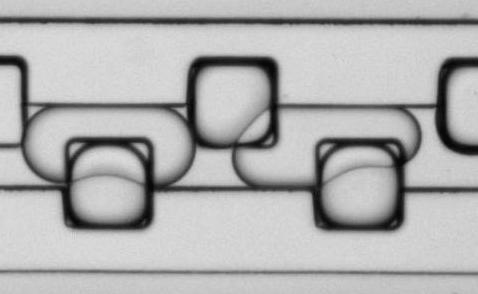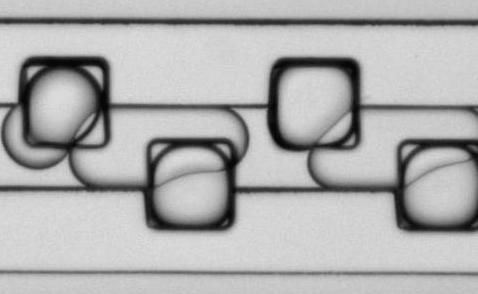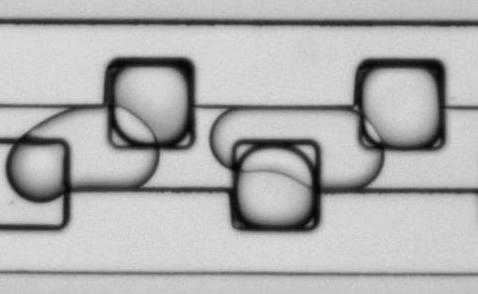
Figure 9: Microfluidic droplet handling. Aqueous droplets containing CaCl2, stabilized with a block co-polymer detergent, and suspended in a fluorous phase are loaded into storage wells (edge length 100 µm) in this time-lapse movie.NG3

Figure 10: ACC formation and crystallization. Montage of time-lapse movies of droplets supersaturated for calcium carbonate during a nucleation assay. Note that while the time between frames is constant at 5 minutes, the frame rate at which this video plays is variable. Images were recorded using polarized light contrast. The initial, non-birefringent precipitate is ACC. Nucleation and growth of faceted, birefringent crystals occurs under dissolution of ACC.
For pure ACC, we find a steady-state rate that is consistent with classical nucleation theory (~1 nucleus cm-3 s-1).3 Notably, this rate is far too low to allow for crystallization in a narrow time window for most intracellular systems. Ba2+ as an inorganic additive, however, has a strong, concentration dependent accelerating effect (Figure 11A).NG4 In addition, we find that the nucleation rate increases with time, consistent with a dynamic lowering of the nucleation barrier (Figure 4B).
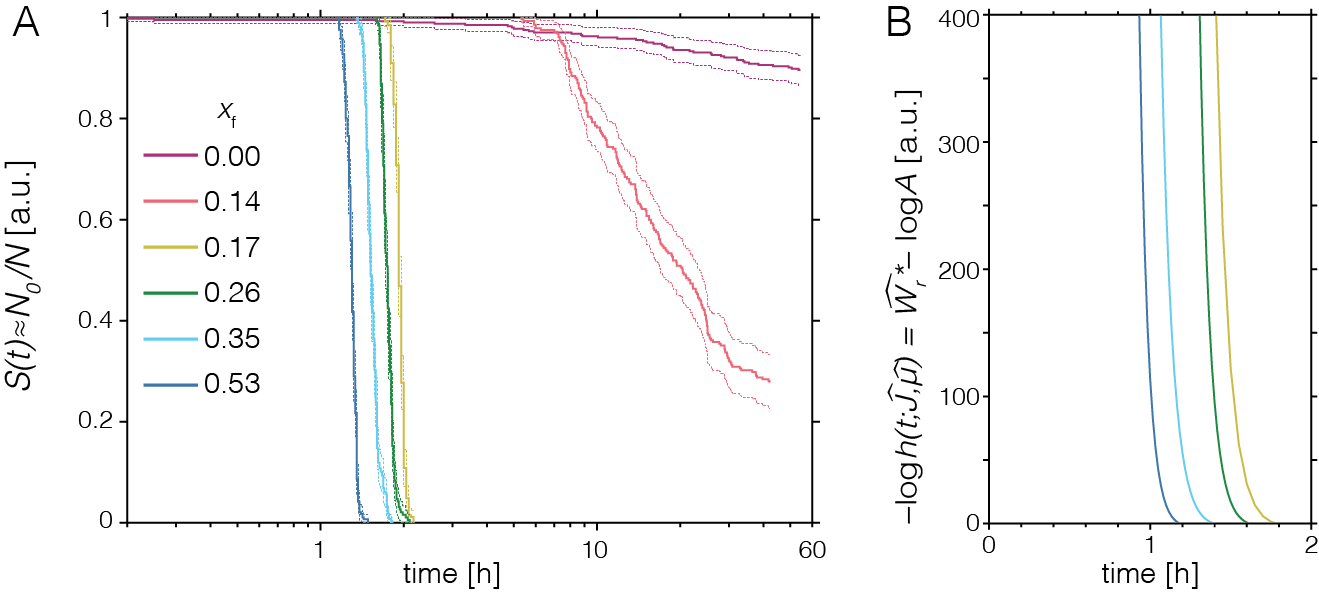
Figure 11: Nucleation rate assay for pure and Ba-substituted ACC. (A) Plot of empirical survivor function (the fraction of droplets in which no crystals have formed, yet) versus logarithmic time for ACC (xf = 0) and amorphous calcium barium carbonate (ACBC) with different compositions (xf indicates cation fraction of barium in starting solution). (B) Plot of the estimate of nucleation barrier versus time. Adapted from Ref. (NG4).
Current projects:
- To improve our ability to extrapolate from 500 pL microfluidic droplets to biological length scales (~0.1 aL – fL), we are working to determine how the nucleation rate scales with droplet volume.
- To improve statistics for the nucleation rate assay, we are developing new microfluidic chips with much larger numbers of droplets and software to efficiently analyze data generated with these.
- To better understand biological control over nucleation and growth, we study the impact of biological macromolecules and inorganic ions on nucleation rates.
Formation of High-Magnesium Calcite in Sea Urchin Teeth
The sea urchin tooth is a fascinating example of the exceptional level of biological control over microstructure and displays a highly hierarchical and functionally graded architecture (Figure 1). Optimized for grazing on hard substrates, its structure comprises both fibrous and platy elements that convey wear resistance, toughness, and self-sharpening to the continuously growing tooth. .
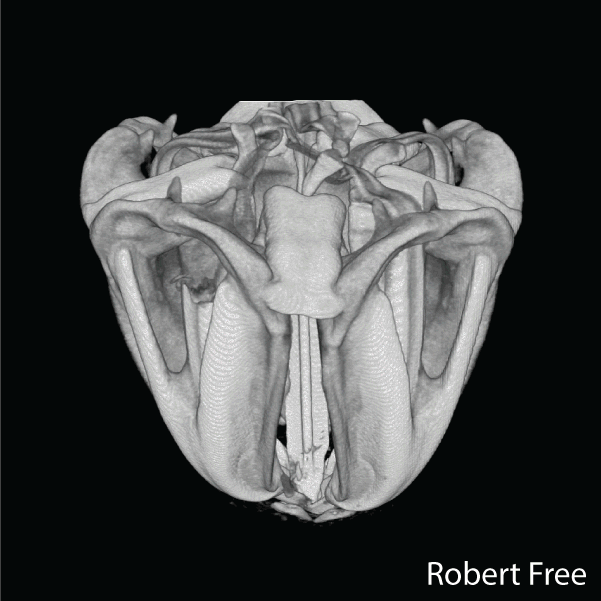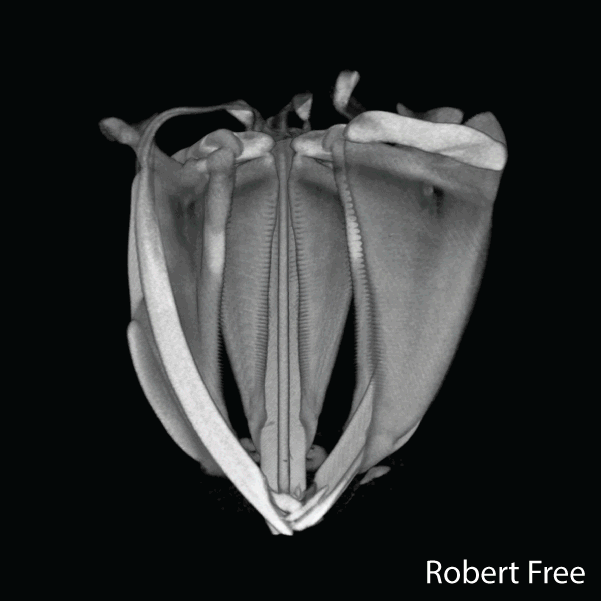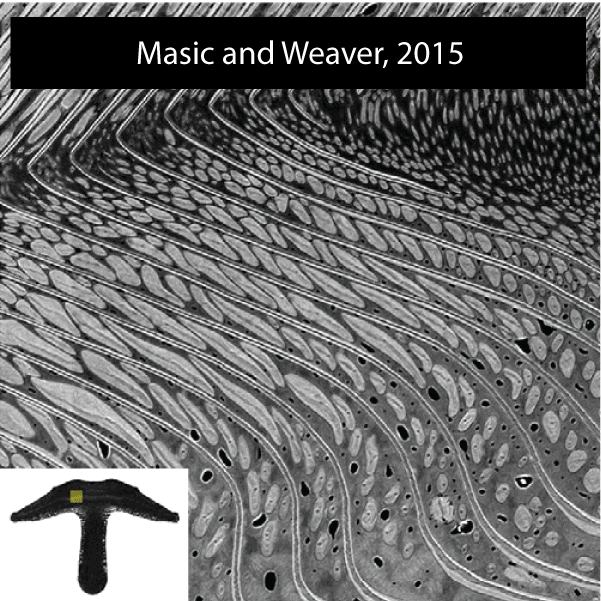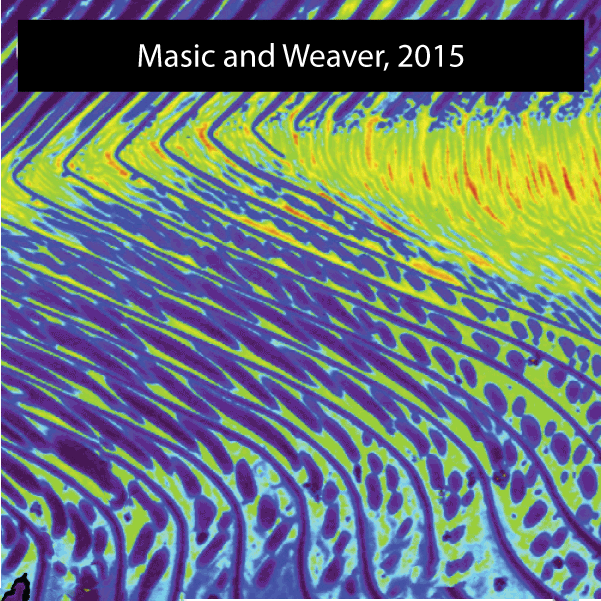
Figure 2.Reconstructions of synchrotron micro computed tomography data sets of the Aristotle’s lantern of Stronglocentrotus. purpuratus (by Robert Free) and microstructure of tooth of Strongylocentrotus franciscanus (adapted from Ref(1).
A unique aspect is that distinct microstructural elements in the tooth differ dramatically in the amount of magnesium incorporated into the calcite (CaCO3) lattice.1,2 Specifically, while in the primary and secondary plates the cation fraction of Mg in high magnesium calcite (HMC) is 13 at%, in the very high magnesium calcite (VHMC) columns between the plates the cation fraction can reach 33 at%. The concentration of Mg2+ in the columns thus far exceeds the solubility limit in calcite and is thought to significantly contribute the overall mechanical function by increasing the hardness. However, VHMC currently cannot be synthesized in the laboratory. Our long-term goal is to elucidate the mechanism that sea urchins use to create Mg-substituted calcite with compositions very far from equilibrium, and to use this understanding to develop bio-inspired syntheses.
We previously identified a novel set of proline+alanine-rich phospho-proteins (PARPs) that are unique to the sea urchin tooth and do not occur in other sea urchin biominerals where Mg levels are lower (3) Our working hypothesis is that PARPs are part of the molecular machinery that enables VHMC synthesis.
Current projects:
- Map the distribution of PARPs across the sea urchin tooth.
- Establish PARP function in vitro
- Dissect role of PARPs in vivo using morpholino antisense oligonucleotide knock-down
References
[1] J. Wilcock, C. Perry, R. Williams, A. Brook, Proceedings of the Royal Society of London, Series B: Biological Sciences 1989, 203.
[2] B. S. Twining, S. B. Baines, N. S. Fisher, J. Maser, S. Vogt, C. Jacobsen, A. Tovar-Sanchez, S. A. Sanudo-Wilhelmy, Analytical Chemistry 2003, 75, 3806.
[3] C. Fahrni, Current opinion in chemical biology 2007, 11, 121.
[4] M. D. Hall, R. A. Alderden, M. Zhang, P. J. Beale, Z. H. Cai, B. Lai, A. P. J. Stampfl, T. W. Hambley, Journal of Structural Biology 2006, 155, 38.
[5] P. J. Endres, K. W. MacRenaris, S. Vogt, M. J. Allen, T. J. Meade, Molecular Imaging 2006, 5, 485.
[6] L. Finney, S. Mandava, L. Ursos, W. Zhang, D. Rodi, S. Vogt, D. Legnini, J. Maser, F. Ikpatt, O. I. Olopade, D. Glesne, Proceedings of the National Academy of Sciences of the United States of America 2007, 104, 2247.
[7] D. Glesne, S. Vogt, J. Maser, D. Legnini, E. Huberman, Journal of Structural Biology 2006, 155, 2.
Home | Contact Us | Sites A-Z









